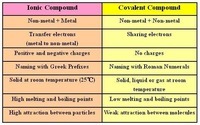
Naming Ionic and Covalent Compounds
Flashcard
•
Other Sciences
•
KG - University
•
Practice Problem
•
Hard

Wayground Content
FREE Resource
Student preview

8 questions
Show all answers
1.
FLASHCARD QUESTION
Front
An ionic compound of calcium and chlorine would be named
Back
calcium chloride.
2.
FLASHCARD QUESTION
Front
A covalent compound made of one sulfur and two oxygen atoms would be named
Back
sulfur dioxide.
3.
FLASHCARD QUESTION
Front
An ionic compound made of copper (Cu2+) and oxygen would be named
Back
copper(II) oxide.
4.
FLASHCARD QUESTION
Front
The covalent compound N2O5 would be named
Back
dinitrogen pentoxide.
5.
FLASHCARD QUESTION
Front
Because the overall charge in a compound must be ____________, the charge of iron in Fe2O3 can be calculated as 3+.
Back
3+
6.
FLASHCARD QUESTION
Front
In the compound TiO2, titanium has a charge of
Back
4+
7.
FLASHCARD QUESTION
Front
The chemical formula for an ionic compound of aluminum and chlorine is
Back
AlCl3.
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

10 questions
Novice Lesson 2 Practice Words
Flashcard
•
KG

10 questions
A lovagokról
Flashcard
•
9th Grade

6 questions
Class rules
Flashcard
•
5th Grade

7 questions
Glossary IRL to revise
Flashcard
•
University

9 questions
Vocabulary Flashcards
Flashcard
•
5th Grade

10 questions
Technology vocabulary
Flashcard
•
University

10 questions
Biomolecules Review
Flashcard
•
9th Grade

10 questions
WATER BODIES
Flashcard
•
6th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

54 questions
Analyzing Line Graphs & Tables
Quiz
•
4th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade
Discover more resources for Other Sciences

20 questions
Place Value
Quiz
•
KG - 3rd Grade

6 questions
3.3 Magnets
Quiz
•
KG

20 questions
Ch. 7 Quadrilateral Quiz Review
Quiz
•
KG - University

12 questions
HOMOPHONES
Lesson
•
KG - 4th Grade

10 questions
Long i- igh, ie, and y Quiz
Quiz
•
KG - 3rd Grade

12 questions
Quarter Past, Half Past, and Quarter To
Quiz
•
KG - 12th Grade

20 questions
Capitalization in sentences
Quiz
•
KG - 4th Grade

14 questions
Reference Sources
Lesson
•
KG - 3rd Grade