
Adv CHEM Unit 11 Day 4 and Day 5 Review
Quiz
•
Chemistry
•
10th Grade
•
Medium
Standards-aligned
Patrick Boylan
Used 318+ times
FREE Resource
Enhance your content in a minute
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt
Identify the products of the chemical equation
3 LiOH + H3PO4 →
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
2.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt
The net ionic equation for ALL aqueous reaction of strong acids and strong soluble bases that form soluble salts is
3.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt
What acid and what base would you choose to prepare the salt potassium chlorate?
4.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
A 50.0 mL sample of Ca(OH)2 is neutralized by 300.0 mL of a 0.50M HCl solution. Calculate the molarity of the Ca(OH)2 solution.
5.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt
How many moles of Ca(OH)2 are needed to neutralize three moles of HCl?
6.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt
For the acid-base titration combination of H2SO4 with 1.96 mol Al(OH)3, find the number of moles of H2SO4 that would be the chemically equivalent amount of Al(OH)3.
7.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt
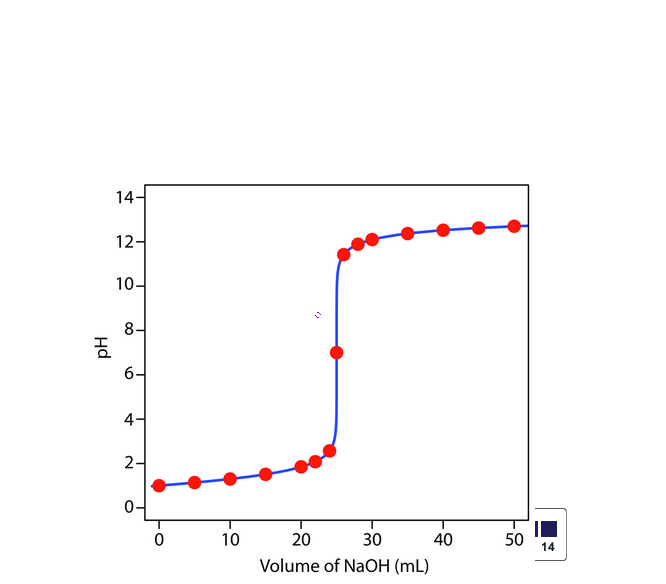
If the concentration of NaOH used is 0.2M, then how many moles of NaOH were used to reach the equivalence point?
Tags
NGSS.HS-PS1-5
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

10 questions
7.0 KADAR TINDAK BALAS
Quiz
•
4th Grade - University

10 questions
chapter 1 quiz 1
Quiz
•
10th Grade

10 questions
Seatwork #2
Quiz
•
9th - 12th Grade

10 questions
Stéréochimie
Quiz
•
1st - 12th Grade

10 questions
Metals Trivia
Quiz
•
9th - 10th Grade

10 questions
Ions
Quiz
•
10th Grade

12 questions
Scientific Skills #5
Quiz
•
7th - 11th Grade

10 questions
Balancing Chemical Equation
Quiz
•
9th - 10th Grade
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade
