
Rate of reaction 1
Quiz
•
Chemistry
•
5th Grade
•
Practice Problem
•
Hard
Standards-aligned
Used 621+ times
FREE Resource
Enhance your content in a minute
13 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
What is the meaning of the rate of reaction?
Decrease in amount of product
Decrease in amount of product against time
Increase in amount of products against time
Increase in amount of reactants against time
Tags
NGSS.HS-PS1-5
2.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which unit is correct for the rate of reaction?
g mol-1
g min-1
mol dm-3
kJ mol-1
Tags
NGSS.HS-PS1-5
3.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which of the following has the lowest rate of reaction?
Combustion of ethanol
Fermentation of glucose
Oxidation of magnesium
Precipitation of silver chloride
Tags
NGSS.HS-PS1-5
4.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which reactions has the highest rate of reaction?
Rusting of water pipe
Photosynthesis in green plant
Burning of a small piece of charcoal in the air
Formation of stalactites and stalagmites
Tags
NGSS.HS-PS1-5
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which process has the highest rate of reaction?
Rusting
Respiration
Combustion
Photosynthesis
Tags
NGSS.HS-PS1-5
6.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
The following equation represents a chemical equation.
CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
Which graph shows the correct change in mass of reactant used in excess against time?


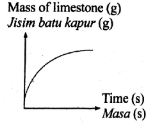
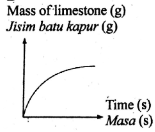
Tags
NGSS.HS-PS1-7
7.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
The rate of reaction for the decomposition of hydrogen peroxide decreases with time because
product of reaction decreases
temperature of hydrogen peroxide decreases
volume of hydrogen peroxide decreases
concentration of hydrogen peroxide decreases
Tags
NGSS.HS-PS1-5
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

10 questions
C3 MOLE Q1
Quiz
•
1st - 5th Grade

10 questions
Random Trivia
Quiz
•
3rd - 6th Grade

15 questions
GRADE 4 PROPERTIES OF MATTER
Quiz
•
4th - 5th Grade

17 questions
Day of the Grandparents
Quiz
•
3rd - 12th Grade

14 questions
6.5 Vocabulary Science
Quiz
•
5th - 6th Grade

9 questions
sulfur cycle
Quiz
•
1st Grade - Professio...

11 questions
Heat Of Neutralisation
Quiz
•
1st - 10th Grade

10 questions
CPR
Quiz
•
1st - 7th Grade
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade
Discover more resources for Chemistry

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade

10 questions
Context Clues
Quiz
•
5th Grade

10 questions
Christmas Trivia for Kids
Quiz
•
5th Grade

21 questions
Christmas Movies
Quiz
•
5th Grade

