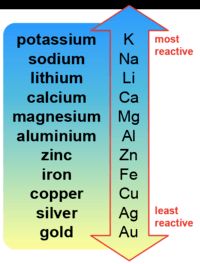
Reactivity Series (Year 8)
Quiz
•
Chemistry, Science
•
8th - 10th Grade
•
Practice Problem
•
Medium
+2
Standards-aligned
Ker GAN
Used 1K+ times
FREE Resource
Enhance your content in a minute
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which of the following is MOST reactive?
Magnesium
Lead
Tin
Copper
Zinc
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which metal is more reactive than calcium?
magnesium
potassium
silver
aluminum
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
How can you tell that a metal is reacting with water?
Tags
NGSS.MS-PS1-2
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Choose the least reactive metal
calcium
magnesium
iron
copper
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Choose the most reactive metal
calcium
magnesium
iron
copper
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which of the following indicates a chemical change?
Tags
NGSS.MS-PS1-2
7.
MULTIPLE SELECT QUESTION
30 sec • 1 pt
Which are the signs of chemical change?
Change in size
Colour change
Precipication
Change in state
Effervescence / Gas produced
Tags
NGSS.MS-PS1-2
NGSS.MS-PS1-5
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

18 questions
Fiche 41-42 Adaptation et évolution
Quiz
•
7th - 8th Grade

14 questions
Specific heat and calorimetry quiz
Quiz
•
10th Grade

20 questions
Química 1
Quiz
•
10th Grade

19 questions
Đơn chất - Hợp chất
Quiz
•
8th - 9th Grade

12 questions
Are You Ready for 8th Grade Science?
Quiz
•
6th - 8th Grade

12 questions
rumus empiris, molekul, dan kristal
Quiz
•
10th Grade

12 questions
Forces
Quiz
•
6th - 8th Grade

15 questions
Penilaian Harian II X IPA (Hukum-Hukum Dasar Kimia)
Quiz
•
10th - 12th Grade
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade
Discover more resources for Chemistry

17 questions
Balancing Equations
Quiz
•
6th - 8th Grade

6 questions
Chemical Formulas
Lesson
•
6th - 8th Grade

20 questions
Ionic Compound Nomenclature
Quiz
•
9th - 12th Grade

31 questions
Science of Christmas
Quiz
•
8th Grade

42 questions
Chemistry Final Exam Review
Quiz
•
9th - 12th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade


