
Reaction Rates
Authored by Jennifer Scott-Burns
Chemistry
9th - 12th Grade
NGSS covered
Used 232+ times

AI Actions
Add similar questions
Adjust reading levels
Convert to real-world scenario
Translate activity
More...
Content View
Student View
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
A substance that increases speed of chemical reaction without being being changed is called:
Tags
NGSS.HS-PS1-5
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Increase in temperature of the reactants can do one of the following
Tags
NGSS.HS-PS1-5
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Grinding a seltzer tablet into powder increases the rate of reaction due to increased
Tags
NGSS.HS-PS1-5
4.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
This slows down or even stops a chemical reaction.
5.
MULTIPLE SELECT QUESTION
30 sec • 1 pt
Which factors affect the rate of a reaction?
temperature
concentration
surface area
pressure
catalyst
Tags
NGSS.HS-PS1-5
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does a higher concentration increase the rate of reaction?
it increases the amount of reactants
it lowers the activation energy
it increases the energy of particle collisions
it increases the frequency of particle collisions
Tags
NGSS.HS-PS1-5
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which graph shows the effect of increasing temperature on the rate of reaction of calcium carbonate with dilute hydrochloric acid?
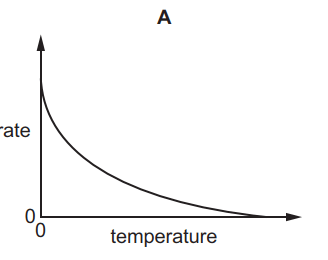
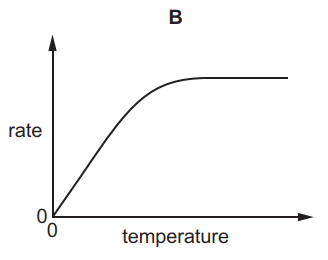
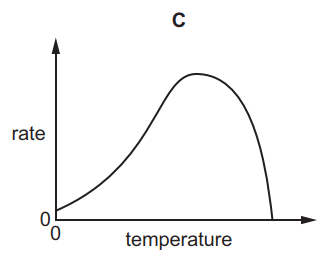
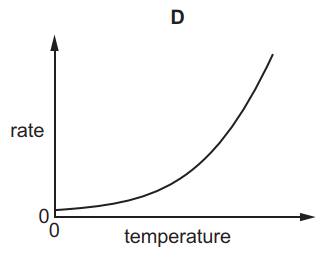
Tags
NGSS.HS-PS1-5
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

10 questions
Atomic structure
Quiz
•
KG - Professional Dev...

8 questions
Average Atomic Mass
Quiz
•
10th Grade

10 questions
Naming Acids
Quiz
•
9th - 12th Grade

10 questions
S - Block Elements (Alkali Metals)
Quiz
•
11th Grade - Professi...

10 questions
Preparing Common Salt
Quiz
•
8th - 10th Grade

10 questions
Petroleum and Its Products
Quiz
•
9th Grade

10 questions
Year 10 Chemistry Term 1 Revision
Quiz
•
10th Grade

10 questions
Mathematics for Chemistry - II
Quiz
•
12th Grade
Popular Resources on Wayground

7 questions
History of Valentine's Day
Interactive video
•
4th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

15 questions
Valentine's Day Trivia
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 8 Stoichiometry Review
Quiz
•
10th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

19 questions
Stoichiometry, Limiting Reactants, and Percent Yield
Quiz
•
10th Grade

20 questions
Stoichiometry Practice
Quiz
•
12th Grade

10 questions
Formative 3BD: Ionic Bonds
Quiz
•
9th Grade

15 questions
Balancing Chemical Equations
Quiz
•
10th - 12th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

10 questions
Identifying types of reactions
Quiz
•
9th - 12th Grade
