
Strengths of Acids and Bases
Quiz
•
Chemistry
•
11th - 12th Grade
•
Medium
Standards-aligned

Amany Fahmy
Used 142+ times
FREE Resource
Student preview

20 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt

Which of the following best approximates the Ka value for this weak acid?
1 x 10–3
1 x 10–4
1 x 10–5
1 x 10–6
2.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which of the following best represents a solution of H2SO4 in water?
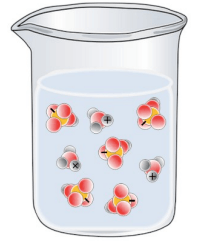
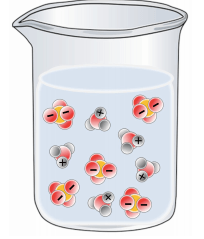
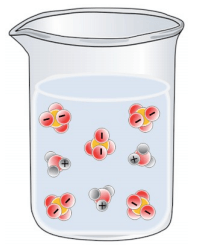
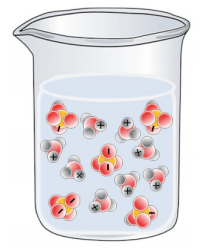
Tags
NGSS.HS-PS1-3
3.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
HSO4¯ + H2O ↔ H3O+ + SO4 2¯ In the equilibrium represented above, the species that act as bases include which of the following?
HSO4-
H2O
SO4 2¯
H2O and SO4 2¯
4.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Given the equation:
H2SO4 + H2O ↔ H3O+ + HSO4- 1
What is the conjugate acid?
H2SO4
H2O
H3O+
HSO4- 1
5.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which compound is the conjugate base?
HCO3- + HCl ==> H2CO3 + Cl-
Which compound is the conjugate base?
HCO3-
HCl
H2CO3
Cl-
6.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt
Which of the following is transferred between a conjugate acid-base pair?
an electron
a proton
a neutron
a OH- ion
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When a hydrogen ion is lost from an acid, the resulting particle is called its conjugate base. Which of the following particles would be the conjugate base for the acid HSO4–?
H2SO4
SO42–
SO4–
HSO4
Create a free account and access millions of resources
Popular Resources on Wayground

25 questions
Equations of Circles
Quiz
•
10th - 11th Grade

30 questions
Week 5 Memory Builder 1 (Multiplication and Division Facts)
Quiz
•
9th Grade

33 questions
Unit 3 Summative - Summer School: Immune System
Quiz
•
10th Grade

10 questions
Writing and Identifying Ratios Practice
Quiz
•
5th - 6th Grade

36 questions
Prime and Composite Numbers
Quiz
•
5th Grade

14 questions
Exterior and Interior angles of Polygons
Quiz
•
8th Grade

37 questions
Camp Re-cap Week 1 (no regression)
Quiz
•
9th - 12th Grade

46 questions
Biology Semester 1 Review
Quiz
•
10th Grade