
Rate of reaction/Kadar tindakbalas
Quiz
•
Chemistry
•
10th Grade
•
Practice Problem
•
Hard
+1
Standards-aligned
ROZALAWATI Moe
Used 33+ times
FREE Resource
Enhance your content in a minute
50 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which of the following process has the highest rate of reaction?
Antara yang berikut, proses yang manakah mempunyai kadar tindak balas
yang paling tinggi?
Rusting
Pengaratan
Respiration
Pernafasan
Combustion
Pembakaran
Photosynthesis
Fotosintesis
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which of the following is the characteristic of catalyst?
Antara yang berikut yang manakah adalah sifat mangkin?
Catalyst used only in solid form.
Mangkin digunakan hanya dalam bentuk pepejal.
Catalyst increases the quantity of product.
Mangkin meningkatkan kuantiti hasil tindak balas
Physical state of catalyst is unchanged during reaction.
Keadaan fizikal mangkin tidak berubah semasa tindak balas.
The quantity of catalyst remains the same after the reaction.
Kuantiti mangkin tetap sama selepas tindak balas.
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Diagram shows the energy profile diagram of a reaction.
Rajah menunjukkan gambar rajah profil tenaga bagi suatu tindak balas.
Based on the Collision Theory, which statement explains the changing of curve I to curve II?
Berdasarkan Teori Perlanggaran, pernyataan manakah menerangkan perubahan lengkungan I kepada lengkungan II?
The total surface area of the solid reactants increases
Jumlah luas permukaan pepejal bahan tindak balas meningkat
The kinetic energy of the particles of reactant decreases
Tenaga kinetik zarah-zarah bahan tindak balas berkurangan
The number of mole per unit volume of particles increases
Bilangan mol per unit isipadu zarah-zarah meningkat
The activation energy of the reaction decreases
Tenaga pengaktifan tindak balas berkurangan
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Why does a higher temperature increase the rate of a reaction?
it increases both the frequency and energy of particle collisions
it only increases the frequency of particle collisions
it only increases the energy of particle collisions
it reduces the activation energy of the reaction
Tags
NGSS.HS-PS1-5
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Grinding a effervescent tablet into powder increases the rate of reaction due to increased
concentration
surface area
temperature
reactants
Tags
NGSS.HS-PS1-5
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Adding a catalyst ___________ the activation energy.
Raises
Lowers
Doesn't affect
Inreases
Tags
NGSS.HS-PS1-5
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which graph shows the effect of increasing temperature on the rate of reaction of calcium carbonate with dilute hydrochloric acid?
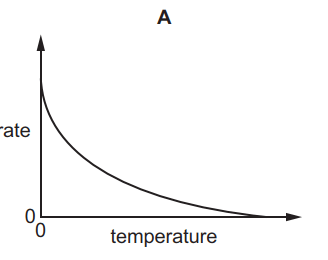
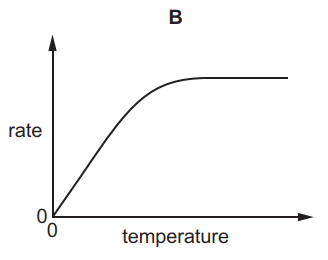
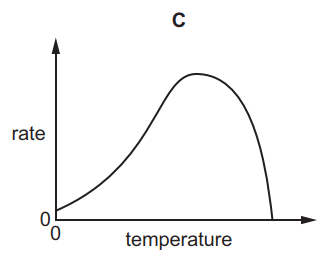
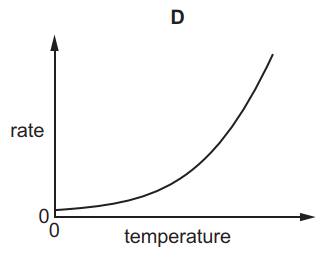
Tags
NGSS.HS-PS1-5
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

50 questions
Periodic table and periodic properties
Quiz
•
10th - 11th Grade

51 questions
Midterm Review (Unit 00-03)
Quiz
•
10th Grade

51 questions
5th grade Science STAAR Review
Quiz
•
KG - University

48 questions
Classification of Matter
Quiz
•
9th - 12th Grade

50 questions
IGCSE Chemistry multiple choice
Quiz
•
10th - 11th Grade

46 questions
AP Chem Unit 2 Review
Quiz
•
10th - 12th Grade

50 questions
Chemistry Yr 9
Quiz
•
9th - 10th Grade

46 questions
Periodic Table Review Quiz
Quiz
•
10th Grade
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade



