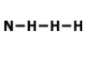Covalent Bonding
Authored by Remy Magbiray
Chemistry
12th Grade - University
NGSS covered
Used 35+ times

AI Actions
Add similar questions
Adjust reading levels
Convert to real-world scenario
Translate activity
More...
Content View
Student View
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 12 pts

Which of the following is the best representation of a molecule of fluorine (F2)?
A
B
C
D
Tags
NGSS.MS-PS1-1
2.
MULTIPLE CHOICE QUESTION
20 sec • 12 pts
Which would be the only situation where valence electrons would be shared evenly?
metals and nonmetals
different nonmetals
nonmetals of the same element
metalloids of the same element
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt

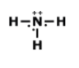
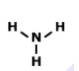
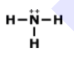
What is the name of this Compound
P2O5
Phosphorous PentaOxide
Diphosphorous pentoxide
Phosphourous Oxide
4.
FILL IN THE BLANK QUESTION
30 sec • 1 pt

What is the formula for this Compound
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Generally, atoms form bonds so that _______________.
Each atom loses its electrons
Each atom has a stable electron configuration
Each atom has an unstable electron configuration
Each atom gains electrons
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
6.
MULTIPLE CHOICE QUESTION
20 sec • 1 pt
Identify the following compound as ionic or covalent: CF4
ionic
covalent
Tags
NGSS.HS-PS1-2
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt

In the polar covalent molecule of hydrogen chloride (HCl) - the chlorine atom has
a lesser pull on electrons and therefore become partially positive charge.
has a greater pull and therefore become partially negative charge.
has no effect on charge because it is neutral.
a lesser effect on valence electrons.
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Kimia- Kaca
Quiz
•
10th - 12th Grade

10 questions
Ikatan Kimia
Quiz
•
University

10 questions
Coordination Chemistry
Quiz
•
University

15 questions
PH-PHR 327 Quiz No. 4 SS 2022-2023
Quiz
•
University

20 questions
LATIHAN US KIMIA SMA
Quiz
•
10th - 12th Grade

20 questions
Inorganic Chemistry: p - block elements
Quiz
•
University

12 questions
Nitrogen and ammonia
Quiz
•
11th Grade - University

12 questions
ANACHE24A Post Lecture Quiz - 032223
Quiz
•
University
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
Energy Transformations
Quiz
•
9th - 12th Grade

24 questions
Identifying Types of Chemical Reactions
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

24 questions
Unit 4 Test Practice: Bonding and Properties
Quiz
•
10th - 12th Grade

21 questions
Introduction to Moles
Lesson
•
9th - 12th Grade

24 questions
ERHS Chem - Chapter 7 Chemical Reactions
Quiz
•
10th - 12th Grade

13 questions
Balanced Eqns & Cons of Mass
Quiz
•
9th - 12th Grade