
Practice Problems Unit 4 Lesson 1
Quiz
•
Chemistry
•
11th Grade - University
•
Practice Problem
•
Medium
+1
Standards-aligned
Ryan McCluskey
Used 4+ times
FREE Resource
Enhance your content in a minute
25 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt
What is the correct Lewis Dot Structure for ammonia NH3
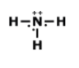
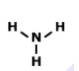
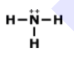
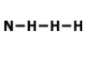
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
2.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt
Which is the correct lewis structure for carbon dioxide?




Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
3.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt

What is the formal charge on the oxygen atom at the top of this Lewis dot structure?
0
-1
+1
-3
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
4.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt

What is the formal charge on the phosphorus atom in this Lewis dot structure?
0
-1
+1
-3
5.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt

What is the formal charge on the bromine atom in this Lewis dot structure?
-1
+1
-2
+2
Tags
NGSS.HS-PS1-1
6.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt
When forming bonds metals tend to __________.
Lose electrons forming positive ions
Lose electrons forming negative ions
Gain electrons forming positive ions
Gain electrons forming negative ions
7.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt
How many total valence electrons are used in the lewis structure for H2O?
8
18
17
7
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

20 questions
Inorganic Chemistry: p - block elements
Quiz
•
University

21 questions
ELECTRON ARRANGEMENT
Quiz
•
10th - 12th Grade

20 questions
PRE-FINAL TEST - GENCHEM 1 (Part 1)
Quiz
•
11th Grade

20 questions
sec 2 lesson 4
Quiz
•
11th Grade

20 questions
Term 1 revision (Gr12)
Quiz
•
12th Grade

20 questions
Chemical Nomenclature
Quiz
•
10th - 11th Grade

20 questions
LATIHAN US KIMIA SMA
Quiz
•
10th - 12th Grade

21 questions
Which structure and particles?
Quiz
•
12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
Energy Transformations
Quiz
•
9th - 12th Grade

17 questions
Reaction Rates
Quiz
•
11th Grade

24 questions
Identifying Types of Chemical Reactions
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

24 questions
Unit 4 Test Practice: Bonding and Properties
Quiz
•
10th - 12th Grade

21 questions
Introduction to Moles
Lesson
•
9th - 12th Grade

24 questions
ERHS Chem - Chapter 7 Chemical Reactions
Quiz
•
10th - 12th Grade


