
REACTION RATES
Authored by CARL RODGERS
Chemistry
9th Grade
Used 8+ times

AI Actions
Add similar questions
Adjust reading levels
Convert to real-world scenario
Translate activity
More...
Content View
Student View
12 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
A substance that increases speed of chemical reaction without being being changed is called:
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Increase in temperature of the reactants can do one of the following
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Grinding a seltzer tablet into powder increases the rate of reaction due to increased
4.
MULTIPLE SELECT QUESTION
30 sec • 1 pt
Which factors affect the rate of a reaction?
temperature
concentration
surface area
pressure
catalyst
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does a higher concentration increase the rate of reaction?
it increases the amount of reactants
it lowers the activation energy
it increases the energy of particle collisions
it increases the frequency of particle collisions
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which graph shows the effect of increasing temperature on the rate of reaction of calcium carbonate with dilute hydrochloric acid?
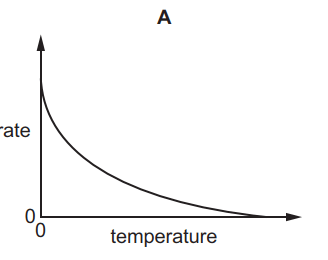
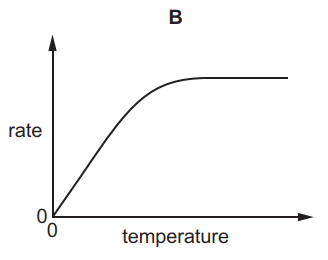
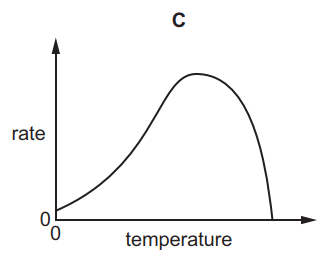
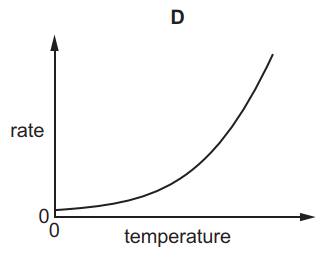
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does a higher concentration increase the rate of reaction?
it increases the amount of reactants
it lowers the activation energy
it increases the energy of particle collisions
it increases the frequency of particle collisions
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

14 questions
Solution, Suspention & Colloidal
Quiz
•
8th - 10th Grade

16 questions
C10.1 GCSE Reactions of Alkenes AQA 8462
Quiz
•
9th - 11th Grade

10 questions
Naming Acids
Quiz
•
9th - 12th Grade

15 questions
The Discovery of the Atom
Quiz
•
7th - 9th Grade

15 questions
Year 9 chemistry revision (fun revise!)
Quiz
•
9th Grade

17 questions
8th grade science STAAR review 1
Quiz
•
KG - University

17 questions
Atom Scientists
Quiz
•
9th - 12th Grade

15 questions
Gas Laws and UNITS
Quiz
•
8th - 10th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

10 questions
Probability Practice
Quiz
•
4th Grade

15 questions
Probability on Number LIne
Quiz
•
4th Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

6 questions
Appropriate Chromebook Usage
Lesson
•
7th Grade

10 questions
Greek Bases tele and phon
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Predicting Products
Quiz
•
9th - 12th Grade

11 questions
Balancing Chemical Equations
Lesson
•
9th Grade

10 questions
Exploring Types of Chemical Reactions
Interactive video
•
6th - 10th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

10 questions
Exploring Ionic and Covalent Bonding Concepts
Interactive video
•
6th - 10th Grade

7 questions
GCSE Chemistry - Balancing Chemical Equations #4
Interactive video
•
9th - 10th Grade

10 questions
Identifying types of reactions
Quiz
•
9th - 12th Grade

10 questions
Identifying Types of Chemical Reactions
Interactive video
•
6th - 10th Grade