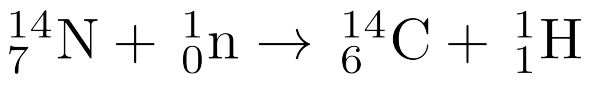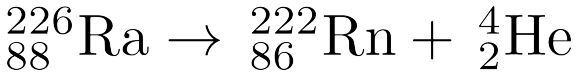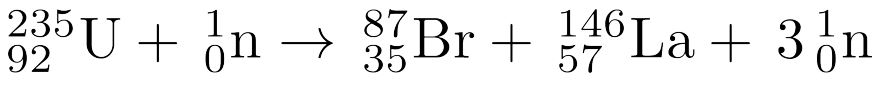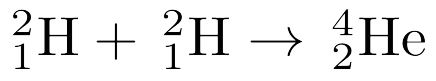Test Review #3- Nuclear Chemistry
Quiz
•
Chemistry
•
9th - 12th Grade
•
Practice Problem
•
Medium
Standards-aligned
Jeffrey Pollack
Used 62+ times
FREE Resource
Enhance your content in a minute
20 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which statement best describes gamma radiation?
It has a mass of 1 and charge of +1
It has a mass of 0 and charge of -1
It has a mass of 0 and charge of 0
It has a mass of 4 and charge of +2
Tags
NGSS.HS-PS1-8
2.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which list of nuclear emissions is arranged in order from the least penetrating power to the greatest penetrating power?
alpha particle, beta particle, gamma ray
alpha particle, gamma ray, beta particle
gamma ray, beta particle, alpha particle
beta particle, gamma ray, alpha particle
3.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
What is the missing isotope that will balance the following nuclear equation?
94Be + 11H → _____ + 42He
Tags
NGSS.HS-PS1-8
4.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt

What particle completes this reaction?
Tags
NGSS.HS-PS1-8
5.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt

Refer to the image. What type of nuclear reaction is shown?
Alpha Decay
Beta Decay
Gamma Emission
Positron Emission
Tags
NGSS.HS-PS1-8
6.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
A radioactive element has a half-life of 2 days. Which fraction represents the amount of an original sample remaining after 6 days?
7.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt

Given the balanced equation representing a nuclear reaction, which phrase identifies and describes this reaction?
Fission, mass converted to energy
Fission, energy converted to mass
Fusion, mass converted to energy
Fusion, energy converted to mass
Tags
NGSS.HS-PS1-8
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

18 questions
Chapter 3 Atoms; Test Review
Quiz
•
10th - 11th Grade

20 questions
Chemistry of Life
Quiz
•
9th Grade

20 questions
Kinetic Theory and States of Matter
Quiz
•
9th - 10th Grade

15 questions
UH Perkembangan Model Atom
Quiz
•
10th Grade

20 questions
Matter Models
Quiz
•
8th - 10th Grade

16 questions
Atomic Structure
Quiz
•
8th - 12th Grade

15 questions
Chemical Reaction Types & Balancing
Quiz
•
10th - 11th Grade

20 questions
Periodic table practice
Quiz
•
12th Grade
Popular Resources on Wayground

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
4:3 Model Multiplication of Decimals by Whole Numbers
Quiz
•
5th Grade

10 questions
The Best Christmas Pageant Ever Chapters 1 & 2
Quiz
•
4th Grade

12 questions
Unit 4 Review Day
Quiz
•
3rd Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

15 questions
Solving Equations with Variables on Both Sides Review
Quiz
•
8th Grade
Discover more resources for Chemistry

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Unit 6-Review The Mole
Quiz
•
11th - 12th Grade

20 questions
Unit 3, Quiz #6 Practice - Types of Covalent
Quiz
•
9th - 12th Grade

17 questions
Protein Synthesis (Protein Synthesis)
Interactive video
•
9th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

10 questions
Exploring Ionic and Covalent Bonding Concepts
Interactive video
•
6th - 10th Grade

148 questions
Fall TEKS Review Chemistry
Quiz
•
9th - 12th Grade

20 questions
Unit 5 - Chemical Reactions Refresh
Quiz
•
9th - 12th Grade