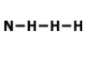Lewis Structures
Quiz
•
Chemistry
•
11th Grade
•
Medium
Gail Chapp
Used 3+ times
FREE Resource
Enhance your content in a minute
13 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
2 mins • 12 pts
When writing Lewis Structures, only __________ electrons are used.
2.
MULTIPLE CHOICE QUESTION
2 mins • 12 pts
Each line in a Lewis Structure represents a bond of __________ electron(s).
3
2
1
4
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to the octet rule most elements need _______ valence electrons.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Hydrogen needs _____ electrons in its valence shell to be stable.
5.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Three pairs of electrons are shared in a
6.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which is the correct molecular structure for carbon dioxide?




7.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt

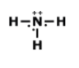
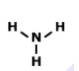
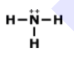
What is the correct formula for this molecule?
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

10 questions
FREE ENERGY
Quiz
•
10th Grade - University

10 questions
Kiểm tra bài cũ hóa 11 bài 13
Quiz
•
11th Grade

12 questions
WODOROTLENKI-quiz
Quiz
•
9th - 12th Grade

17 questions
Загальні властивості металів 11 клас
Quiz
•
1st - 12th Grade

10 questions
Prueba corta #1
Quiz
•
9th - 12th Grade

15 questions
Ароматты көмірсутектер
Quiz
•
9th - 11th Grade

15 questions
hoa 11. ĐIỆN LY
Quiz
•
11th - 12th Grade

15 questions
Intro to Stoichiometry
Quiz
•
10th - 12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
Energy Transformations
Quiz
•
9th - 12th Grade

17 questions
Reaction Rates
Quiz
•
11th Grade

24 questions
Identifying Types of Chemical Reactions
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

24 questions
Unit 4 Test Practice: Bonding and Properties
Quiz
•
10th - 12th Grade

21 questions
Introduction to Moles
Lesson
•
9th - 12th Grade

24 questions
ERHS Chem - Chapter 7 Chemical Reactions
Quiz
•
10th - 12th Grade