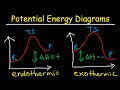
Exploring Potential Energy Diagrams in Chemical Reactions
Interactive Video
•
Science
•
6th - 10th Grade
•
Practice Problem
•
Easy
Standards-aligned
Emma Peterson
Used 8+ times
FREE Resource
Standards-aligned
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the term for the highest point on a potential energy diagram?
Products
Reactants
Transition State
Intermediate
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In an exothermic reaction, how does the energy of the products compare to the reactants?
Energy comparison is not relevant
Products have more energy
Products have less energy
Products have the same energy
Tags
NGSS.HS-PS1-4
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary role of a catalyst in a chemical reaction?
Increase the activation energy
Lower the activation energy
Decrease the energy of products
Increase the energy of reactants
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does adding a catalyst affect the speed of a reaction?
It slows down the reaction
It has no effect on the reaction speed
It speeds up the reaction
It stops the reaction
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In an endothermic reaction, what is the sign of Delta H?
Undefined
Negative
Zero
Positive
Tags
NGSS.HS-PS1-4
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you calculate the forward activation energy?
Products minus reactants
Transition state minus reactants
Reactants minus products
Transition state minus products
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a two-step reaction, what determines the slow step?
The step with the higher activation energy
The step with the intermediate energy
The step with the lower activation energy
The step with the highest product energy
Tags
NGSS.HS-PS1-4
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Science

33 questions
Grade 6 Quarter 3 PMA 5 Review
Quiz
•
6th - 8th Grade

85 questions
Midpoint D1 Review
Quiz
•
8th Grade

20 questions
Rocks and The Rock Cycle
Quiz
•
6th Grade

12 questions
Ecological Succession
Quiz
•
7th Grade

12 questions
Newton's Laws of Motion
Lesson
•
6th - 8th Grade

10 questions
Exploring the Rock Cycle: Types and Formation
Interactive video
•
6th - 8th Grade

10 questions
Exploring the Layers of the Earth
Interactive video
•
6th - 10th Grade

20 questions
Pure Substances & Mixtures
Quiz
•
8th Grade