Acid-Base Chemistry and pH Calculations
Interactive Video
•
Chemistry, Science
•
10th - 12th Grade
•
Hard
Jackson Turner
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why was the hydrolysis equation for aluminum chloride not written in the previous video?
It is a neutral compound.
It is not relevant to the topic.
It is a very complicated equation.
It is a very simple equation.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following ions is considered neutral?
Iron
Aluminum
Chromium
Chloride
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What characteristic do small, highly charged cations share?
They have no charge.
They are always neutral.
They act as bases.
They act as acids.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to water molecules when they surround an aluminum ion in a salt solution?
They form a hydration sphere.
They dissociate completely.
They form a crystal structure.
They become neutral.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
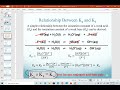
What is the relationship between Ka and Kb for a conjugate acid-base pair?
Ka times Kb equals Kw.
Ka is always greater than Kb.
Ka is always less than Kb.
Ka and Kb are unrelated.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the value of Kw at 25-degrees Celsius?
1.0 times 10 to the minus 21
1.0 times 10 to the minus 14
1.0 times 10 to the minus 7
1.0 times 10 to the minus 10
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you calculate the Kb of an ion if you know the Ka of its conjugate acid?
Subtract Kw from the Ka of the conjugate acid.
Multiply Kw by the Ka of the conjugate acid.
Divide Kw by the Ka of the conjugate acid.
Add Kw to the Ka of the conjugate acid.
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

11 questions
NEASC Extended Advisory
Lesson
•
9th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade