What is a defining characteristic of a pure substance?
Understanding Pure Substances
Interactive Video
•
Sophia Harris
•
Chemistry, Science
•
6th - 8th Grade
•
2 plays
•
Medium
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
It is composed of multiple types of atoms.
It has changing chemical properties.
It is composed of only one type of atom or particle.
It is always a liquid.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is an example of an element?
Air
Gold
Salt
Water
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of atoms make up the element carbon?
Oxygen atoms
Gold atoms
Hydrogen atoms
Carbon atoms
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is a compound?
Gold
Carbon
Water
Oxygen
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the chemical formula for water?
CO2
H2O
O2
NaCl
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What particles make up a water molecule?
One hydrogen atom and one oxygen atom
Two hydrogen atoms and two oxygen atoms
One oxygen atom and two hydrogen atoms
Two oxygen atoms and one hydrogen atom
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the common name for sodium chloride?
Vinegar
Baking soda
Salt
Sugar
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What distinguishes a mixture from a pure substance?
A mixture has changing physical properties.
A mixture is made of only one type of particle.
A mixture is composed of more than one type of particle.
A mixture is always a solid.
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is an example of a mixture?
Gold
Muddy water
Carbon
Pure water
10.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
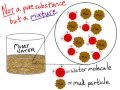
Why is muddy water not considered a pure substance?
It is composed of only one type of particle.
It has a uniform composition.
It contains both water molecules and mud particles.
It is a chemical compound.
Explore all questions with a free account
Similar Resources on Quizizz

11 questions
Chemistry Concepts and Mixtures
Interactive video
•
6th - 8th Grade

11 questions
Composition and Properties of Matter
Interactive video
•
6th - 8th Grade

11 questions
Classifying Matter: Elements, Compounds, and Mixtures Explained
Interactive video
•
6th - 8th Grade

11 questions
Understanding Atomic Structure and Elements
Interactive video
•
6th - 8th Grade

11 questions
Suspensions and Solutions in Chemistry
Interactive video
•
6th - 8th Grade

11 questions
Properties and Classification of Mixtures
Interactive video
•
6th - 8th Grade

11 questions
Matter and Its Mixtures in the Universe
Interactive video
•
6th - 8th Grade

11 questions
Chemical Compounds and Elements
Interactive video
•
6th - 8th Grade
Popular Resources on Quizizz

17 questions
CAASPP Math Practice 3rd
Quiz
•
3rd Grade

20 questions
math review
Quiz
•
4th Grade

21 questions
6th Grade Math CAASPP Practice
Quiz
•
6th Grade

13 questions
Cinco de mayo
Interactive video
•
6th - 8th Grade

20 questions
Reading Comprehension
Quiz
•
5th Grade

20 questions
Types of Credit
Quiz
•
9th - 12th Grade

10 questions
4th Grade Math CAASPP (part 1)
Quiz
•
4th Grade

45 questions
5th Grade CAASPP Math Review
Quiz
•
5th Grade
Discover more resources for Chemistry

13 questions
Bill Nye - Energy
Interactive video
•
6th Grade

20 questions
Acids and Bases
Quiz
•
8th Grade

22 questions
8th Science EOG Review - Chemistry
Quiz
•
7th - 8th Grade

20 questions
Chemical Reactions
Quiz
•
8th Grade

34 questions
Acids and Bases
Quiz
•
8th - 11th Grade

20 questions
Periodic Table Review
Quiz
•
7th Grade

20 questions
States of Matter
Quiz
•
8th Grade

41 questions
Atomic Structure and Periodic Table Unit Review
Quiz
•
8th Grade