Which equations are not included on the equation sheet?
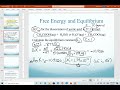
Equilibrium Constants and Delta G
Interactive Video
•
Lucas Foster
•
Chemistry, Science
•
10th - 12th Grade
•
Hard
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Equations 2 and 6
Equations 3 and 5
Equations 1 and 3
Equations 1, 2, and 4
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the main mathematical function used in Equations 1, 2, and 4?
Multiplication
Addition and Subtraction
Division
Exponentiation
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Equation 3 is used to solve for which of the following?
Temperature
Concentration
Volume
Pressure
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is the equilibrium constant related to standard Delta G in Equation 6?
Through addition
Through division
Through subtraction
Through multiplication
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the expected value of K for a reactant-favored system?
Cannot be determined
Less than 1
Equal to 1
Greater than 1
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the common mistake students make when calculating K?
Forgetting to convert units
Forgetting to carry the negative sign
Using the wrong equation
Misplacing decimal points
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of a positive Delta G in terms of reaction spontaneity?
The reaction is at equilibrium
The reaction is non-spontaneous
The reaction rate is increased
The reaction is spontaneous
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the value of Delta G naught used in the final practice problem?
27.1 kilojoules per mole
5.9 kilojoules per mole
10.9 kilojoules per mole
1.79 kilojoules per mole
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the calculated value of Q in the final practice problem?
9.782 x 10^-4
1.79 x 10^-5
5.9 x 10^-3
10.9 x 10^-2
10.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does a positive final Delta G indicate about the direction of the reaction?
It is accelerating
It is at equilibrium
It is moving in reverse
It is moving forward
Explore all questions with a free account
Similar Resources on Quizizz

11 questions
Spontaneity and Thermodynamics Concepts
Interactive video
•
11th - 12th Grade

11 questions
Understanding Delta G and Equilibrium
Interactive video
•
10th - 12th Grade

11 questions
Gibbs Free Energy and Spontaneity
Interactive video
•
10th - 12th Grade

11 questions
Gibbs Free Energy and Its Impact on Chemical Reactions
Interactive video
•
11th - 12th Grade

11 questions
Understanding Free Energy and Equilibrium
Interactive video
•
10th - 12th Grade

11 questions
Delta G and Free Energy Concepts
Interactive video
•
9th - 12th Grade

11 questions
Spontaneity in Chemical Reactions
Interactive video
•
10th - 12th Grade

11 questions
Thermochemistry and Energy Changes
Interactive video
•
10th - 12th Grade
Popular Resources on Quizizz

17 questions
CAASPP Math Practice 3rd
Quiz
•
3rd Grade

20 questions
math review
Quiz
•
4th Grade

21 questions
6th Grade Math CAASPP Practice
Quiz
•
6th Grade

13 questions
Cinco de mayo
Interactive video
•
6th - 8th Grade

20 questions
Reading Comprehension
Quiz
•
5th Grade

20 questions
Types of Credit
Quiz
•
9th - 12th Grade

10 questions
4th Grade Math CAASPP (part 1)
Quiz
•
4th Grade

45 questions
5th Grade CAASPP Math Review
Quiz
•
5th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
Acids and Bases
Quiz
•
10th Grade

20 questions
Stoichiometry Practice
Quiz
•
10th Grade

20 questions
Balancing Chemical Equations
Quiz
•
9th - 11th Grade

20 questions
Balancing Chemical Equations and Types of Reactions
Quiz
•
10th Grade

24 questions
Types of Chemical Reactions
Quiz
•
10th Grade

20 questions
Stoichiometry Practice Quiz
Quiz
•
10th Grade

47 questions
Thermochemistry Review
Quiz
•
10th Grade