
Hazard Symbols
Quiz
•
English, Science
•
5th Grade - University
•
Practice Problem
•
Easy
Karolin
Used 94+ times
FREE Resource
Enhance your content in a minute
22 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
10 sec • 1 pt
What is the correct symbol?
"Corrosive"




2.
MULTIPLE CHOICE QUESTION
10 sec • 1 pt
What is the correct symbol?
"Explosive"




3.
MULTIPLE CHOICE QUESTION
10 sec • 1 pt
What is the correct symbol?
"Oxidizing"




4.
MULTIPLE CHOICE QUESTION
10 sec • 1 pt
What is the correct symbol?
"Biohazard"

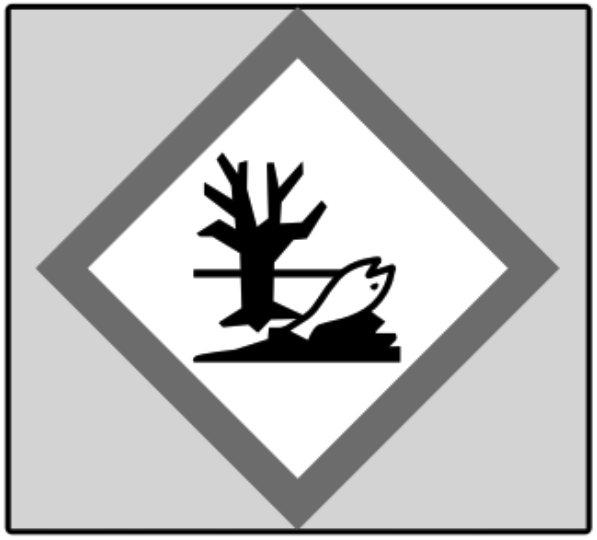

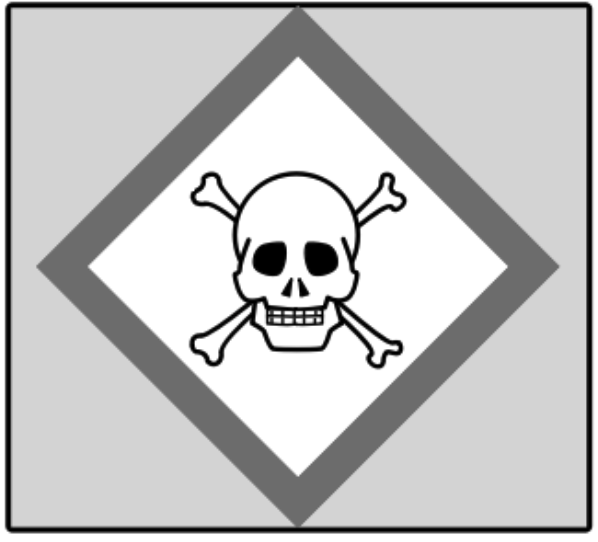
5.
MULTIPLE CHOICE QUESTION
10 sec • 1 pt
What is the correct symbol?
"Acute Toxicity"




6.
MULTIPLE CHOICE QUESTION
10 sec • 1 pt
What is the correct symbol?
"Flammable"




7.
MULTIPLE CHOICE QUESTION
10 sec • 1 pt
What is the correct symbol?
"Radioactive"




Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

20 questions
Elements & compounds
Quiz
•
6th Grade

18 questions
Acids, alkalis and electricity
Quiz
•
6th Grade

19 questions
Chemistry Practice
Quiz
•
8th Grade

19 questions
The USA Quiz 1
Quiz
•
7th - 9th Grade

18 questions
Living in a big city 1
Quiz
•
7th Grade - University

20 questions
1st Year Assessment 1
Quiz
•
6th - 8th Grade

20 questions
End of term exam - Mega Quizizz!
Quiz
•
6th - 9th Grade

20 questions
Daily Test Unit 2
Quiz
•
10th Grade
Popular Resources on Wayground

7 questions
History of Valentine's Day
Interactive video
•
4th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

15 questions
Valentine's Day Trivia
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade
Discover more resources for English

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade

10 questions
Exploring Valentine's Day with Charlie Brown
Interactive video
•
6th - 10th Grade

12 questions
Figurative Language Review
Interactive video
•
5th Grade

20 questions
Prefix and Suffix Review
Quiz
•
3rd - 5th Grade

25 questions
7th Reading STAAR Vocabulary
Quiz
•
6th - 8th Grade

20 questions
Revising & Editing practice
Quiz
•
7th Grade




