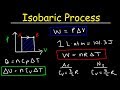
Thermodynamics and Gas Laws
Interactive Video
•
Physics, Chemistry, Science
•
10th - 12th Grade
•
Hard
Olivia Brooks
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formula used to calculate the work done by a gas during an isobaric process?
W = nRT
W = PΔV
W = PV
W = nCvΔT
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In an isobaric process, if a gas expands, what is the sign of the work done?
Positive
Undefined
Zero
Negative
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which law relates the volume and temperature of a gas at constant pressure?
Boyle's Law
Charles's Law
Avogadro's Law
Dalton's Law
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the value of the gas constant R when using liters and atm?
8.3145 J/mol·K
0.08206 L·atm/mol·K
1.013 L·atm/mol·K
22.4 L/mol
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does the temperature change when the volume of a gas doubles at constant pressure?
It triples
It doubles
It remains the same
It halves
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar heat capacity at constant pressure (Cp) for a monoatomic gas?
9/2 R
5/2 R
3/2 R
7/2 R
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following gases is considered monoatomic?
Carbon Dioxide
Helium
Nitrogen
Oxygen
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Thermal Processes and Gas Laws
Interactive video
•
11th - 12th Grade

11 questions
Thermodynamics and the Drinking Bird
Interactive video
•
9th - 12th Grade

11 questions
Thermodynamic Equations and Internal Energy
Interactive video
•
9th - 12th Grade

4 questions
Thermodynamics: Crash Course Physics
Interactive video
•
11th Grade - University

11 questions
Understanding Gases and the Ideal Gas Law
Interactive video
•
10th - 12th Grade

11 questions
Gas Laws and Kinetic Theory Concepts
Interactive video
•
10th - 12th Grade

6 questions
Ideal Gas Law and Its Variations
Interactive video
•
10th - 12th Grade

11 questions
Gas Stoichiometry and Ideal Gas Law Concepts
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Physics

20 questions
Claim Evidence Reasoning
Quiz
•
9th - 12th Grade

14 questions
Distance & Displacement
Quiz
•
11th Grade

17 questions
Free Body Diagrams
Quiz
•
9th - 12th Grade

20 questions
Motion Graphs
Quiz
•
11th - 12th Grade

10 questions
Distance & Displacement
Quiz
•
9th - 12th Grade

19 questions
Graphing Motion Review
Quiz
•
9th - 12th Grade

20 questions
Multiplying/ Dividing Significant Figures
Quiz
•
11th Grade

23 questions
Unit 1 Graphing and Pendulum
Quiz
•
9th - 12th Grade