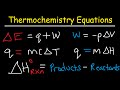
Thermochemistry Concepts and Calculations
Interactive Video
•
Chemistry, Science
•
10th - 12th Grade
•
Medium
Emma Peterson
Used 1+ times
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the sign of Q when heat is released from a system?
Positive
Undefined
Negative
Zero
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If a gas expands from 2 L to 3 L at a pressure of 5 ATM, what is the sign of the work done by the system?
Zero
Negative
Undefined
Positive
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How much energy is required to heat 50 grams of water from 25°C to 75°C?
20,920 J
2,615 J
5,230 J
10,460 J
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the heat of fusion for water in kJ per mole?
4.184 kJ
6 kJ
18 kJ
12 kJ
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a combustion reaction, if 64 grams of oxygen reacts, how many kJ of heat energy will be released?
1,200 kJ
2,400 kJ
960 kJ
480 kJ
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the enthalpy change when 3600 kJ of heat energy is released in a reaction?
99 grams of CO2
792 grams of CO2
198 grams of CO2
396 grams of CO2
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the enthalpy of formation for CO2?
0 kJ/mol
-393 kJ/mol
-286 kJ/mol
-785 kJ/mol
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Thermochemistry Concepts and Applications
Interactive video
•
9th - 10th Grade

6 questions
IIT/JEE Chemistry Practice #26: Enthalpy of Combustion
Interactive video
•
11th Grade - University

11 questions
Chemical Reactions and Energy Changes
Interactive video
•
9th - 12th Grade

6 questions
Thermochemistry: Heat and Enthalpy
Interactive video
•
11th Grade - University

2 questions
IIT/JEE Chemistry Practice #26: Enthalpy of Combustion
Interactive video
•
11th Grade - University

2 questions
Hess's Law and Heats of Formation
Interactive video
•
10th - 12th Grade

11 questions
Enthalpy and Heat of Formation
Interactive video
•
9th - 12th Grade

8 questions
Chemical engineer Robert Coolman - ScienceLives
Interactive video
•
11th Grade - University
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade