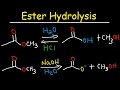
Acid and Base Catalysis Concepts
Interactive Video
•
Chemistry, Science
•
10th Grade - University
•
Hard
Ethan Morris
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are the products when methyl acetate reacts with water and HCl under acidic conditions?
Carboxylic acid and methanol
Acetic acid and ethanol
Ethanol and acetic acid
Methanol and ethyl acetate
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the acid-catalyzed ester hydrolysis, which ion is formed when HCl is added to water?
Na+
H3O+
Cl-
OH-
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
During the acid-catalyzed mechanism, which oxygen in the ester is more likely to be protonated?
The oxygen with no charge
The oxygen with a negative formal charge
The oxygen bonded to carbon
The oxygen with a positive formal charge
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of water in the acid-catalyzed ester hydrolysis mechanism?
Acts as a weak base
Acts as a strong acid
Acts as a nucleophile
Acts as a catalyst
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final product of the acid-catalyzed ester hydrolysis?
Ester
Methanol
Deprotonated carboxylic acid
Protonated carboxylic acid
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the base-catalyzed mechanism, what is the role of hydroxide?
Acts as a catalyst
Acts as an acid
Acts as a solvent
Acts as a nucleophile
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to methoxide in the base-catalyzed ester hydrolysis?
It acts as a nucleophile
It forms a double bond
It attacks the carbonyl carbon
It deprotonates the carboxylic acid
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

8 questions
Dipole Forces
Interactive video
•
11th Grade - University

8 questions
How Cells Got Their Membranes (Maybe) | SciShow News
Interactive video
•
11th Grade - University

8 questions
Schmidt Reaction
Interactive video
•
11th Grade - University

6 questions
The Deal with Protein
Interactive video
•
11th Grade - University

10 questions
Acid-Base Properties of Salts
Interactive video
•
10th - 12th Grade

11 questions
Acid-Base Balance and Disorders
Interactive video
•
10th Grade - University

11 questions
Halogen Bonds and Hedgehog Biology
Interactive video
•
10th Grade - University

6 questions
Stereoisomerism: Unlocking the Secrets of Molecular Twins
Interactive video
•
10th Grade - University
Popular Resources on Wayground

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

20 questions
MINERS Core Values Quiz
Quiz
•
8th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade

10 questions
How to Email your Teacher
Quiz
•
Professional Development

15 questions
Order of Operations
Quiz
•
5th Grade
Discover more resources for Chemistry

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

19 questions
Lewis Dot Structures -Review and Master
Quiz
•
10th Grade

10 questions
Electron Configuration, Orbital Notation, & Dot diagrams
Lesson
•
9th - 12th Grade

10 questions
Intro to Atoms Vocabulary Quiz
Quiz
•
8th - 10th Grade

20 questions
Naming Polyatomic Ionic compounds
Quiz
•
9th - 12th Grade

45 questions
Unit 3: Atomic Assault Summative Review
Quiz
•
11th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade