
REVIEW UNIT 3
Authored by Salwa Ahmad
Science
1st Grade
Used 4+ times

AI Actions
Add similar questions
Adjust reading levels
Convert to real-world scenario
Translate activity
More...
Content View
Student View
20 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What thing is made of metal?




2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt

What are they made of?
They are made of glass.
They are made of concrete
They are made of rubber
They are made of rock.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt

What is it made of?
It is made of fabric
It is made of paper
It is made of concrete
It is made of metal
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
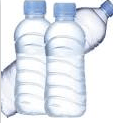
Which object is made of plastic?



5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt

What is this window made of?
It is made of wood and wool.
It is made of wool and glass.
It is made of paper and fabric.
It is made of wood and glass.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt

What is it made of?
It is made of plastic
It is made of glass
It is made of rock
It is made of wood
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt

What are the books made of?
They're made of gold.
They're made of paper.
They're made of glass
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

17 questions
Animals (KG)
Quiz
•
KG - 1st Grade

15 questions
P1 Habitats of the Animals
Quiz
•
1st Grade

15 questions
Animals look like their parents
Quiz
•
1st Grade

19 questions
Weather symbols
Quiz
•
1st - 2nd Grade

18 questions
ทบทวน material
Quiz
•
1st Grade

20 questions
EVS QUIZ
Quiz
•
1st Grade

15 questions
PARTS OF ANIMAL ii
Quiz
•
1st - 2nd Grade

15 questions
States of Matter and Changes of State
Quiz
•
1st - 5th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

10 questions
Probability Practice
Quiz
•
4th Grade

15 questions
Probability on Number LIne
Quiz
•
4th Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

6 questions
Appropriate Chromebook Usage
Lesson
•
7th Grade

10 questions
Greek Bases tele and phon
Quiz
•
6th - 8th Grade
Discover more resources for Science

5 questions
Heating and Cooling Pre-Assessment
Quiz
•
1st Grade

10 questions
Identifying Physical and Chemical Changes
Interactive video
•
1st - 5th Grade

7 questions
Layers of Soil
Interactive video
•
1st - 5th Grade

10 questions
Exploring the 11 Human Body Systems
Interactive video
•
1st - 5th Grade

10 questions
Flow of Energy and Matter in Ecosystems
Interactive video
•
1st - 5th Grade

9 questions
Forces: Push and Pull
Quiz
•
1st Grade

12 questions
Earthquakes, Landslides, and Volcanoes
Quiz
•
1st - 5th Grade

10 questions
Exploring Different Types of Landforms
Interactive video
•
1st - 5th Grade


