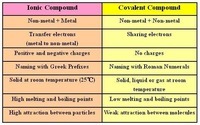
Ionic and Covalent Compounds, Properties and Lewis Structures, Naming and Writing Formulas
Quiz
•
Other Sciences
•
KG - University
•
Hard

Charles Martinez
FREE Resource
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Greenhouse Gases/ Ozone
Quiz
•
6th - 8th Grade

11 questions
Intermolecular Forces and Macroscopic Properties
Quiz
•
10th - 12th Grade

11 questions
Flocabulary: Mixtures & Compounds
Quiz
•
4th - 6th Grade

10 questions
elements
Quiz
•
6th - 8th Grade

15 questions
Greenhouse Gases and Ozone Depletion Review
Quiz
•
6th - 7th Grade

13 questions
8th grade science
Quiz
•
8th Grade

10 questions
Naming Ionic and Covalent Bonds
Quiz
•
KG - University

10 questions
Writing Ionic and Covalent Compounds
Quiz
•
KG - University
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Other Sciences

20 questions
Disney Characters
Quiz
•
KG

18 questions
Hispanic Heritage Month
Quiz
•
KG - 12th Grade

10 questions
Would you rather...
Quiz
•
KG - University

20 questions
Place Value
Quiz
•
KG - 3rd Grade

10 questions
MTSS - Attendance
Quiz
•
KG - 5th Grade

20 questions
Logos
Quiz
•
KG

12 questions
Continents and Oceans
Quiz
•
KG - 8th Grade

12 questions
Doubles and Near Doubles
Quiz
•
KG - 2nd Grade

