
Strength of Acids and Bases Worksheet
Quiz
•
Chemistry
•
11th - 12th Grade
•
Practice Problem
•
Hard
Standards-aligned

Charles Martinez
FREE Resource
Enhance your content in a minute
20 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt

Which of the following best approximates the Ka value for this weak acid?
1 x 10–3
1 x 10–4
1 x 10–5
1 x 10–6
2.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which of the following best represents a solution of H2SO4 in water?
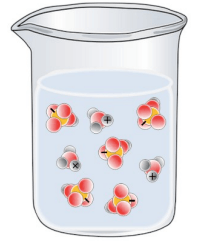
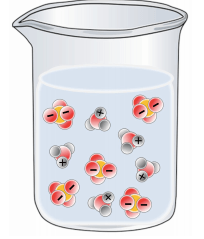
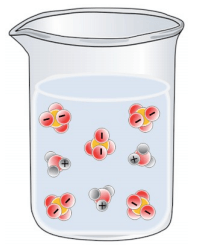
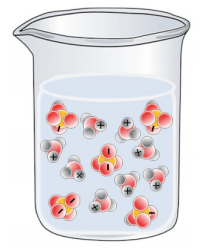
Tags
NGSS.HS-PS1-2
3.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
HSO4¯ + H2O ↔ H3O+ + SO4 2¯ In the equilibrium represented above, the species that act as bases include which of the following?
HSO4-
H2O
SO4 2¯
H2O and SO4 2¯
4.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Given the equation:
H2SO4 + H2O ↔ H3O+ + HSO4- 1
What is the conjugate acid?
H2SO4
H2O
H3O+
HSO4- 1
5.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which compound is the conjugate base?
HCO3- + HCl ==> H2CO3 + Cl-
Which compound is the conjugate base?
HCO3-
HCl
H2CO3
Cl-
6.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt
Which of the following is transferred between a conjugate acid-base pair?
an electron
a proton
a neutron
a OH- ion
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When a hydrogen ion is lost from an acid, the resulting particle is called its conjugate base. Which of the following particles would be the conjugate base for the acid HSO4–?
H2SO4
SO42–
SO4–
HSO4
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

17 questions
BONDING FOR ALEVEL
Quiz
•
12th Grade - University

19 questions
OCR A Chemistry – 5.2.2 Entropy and ∆G
Quiz
•
12th Grade

20 questions
IPC Unit 8: Changes in Matter
Quiz
•
9th - 12th Grade

15 questions
sel volta
Quiz
•
12th Grade

21 questions
Matter in Our Surroundings
Quiz
•
9th - 11th Grade

25 questions
Ionic vs Covalent Bonds
Quiz
•
9th - 12th Grade

20 questions
ASAM BASA
Quiz
•
11th Grade

20 questions
Compounds: Formulas and Names
Quiz
•
12th Grade
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

15 questions
4:3 Model Multiplication of Decimals by Whole Numbers
Quiz
•
5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
The Best Christmas Pageant Ever Chapters 1 & 2
Quiz
•
4th Grade

12 questions
Unit 4 Review Day
Quiz
•
3rd Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade
Discover more resources for Chemistry

20 questions
Unit 6-Review The Mole
Quiz
•
11th - 12th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

21 questions
Unit 6 -The Mole Review
Quiz
•
11th - 12th Grade

20 questions
Naming Compounds: Basic Ionic and Covalent Naming
Quiz
•
9th - 12th Grade

20 questions
Naming Covalent Compounds
Quiz
•
11th Grade

20 questions
Balancing Chemical Equations
Quiz
•
10th - 12th Grade

65 questions
Midterm Review Chem
Quiz
•
9th - 12th Grade

35 questions
Chemistry Semester A Final Review
Quiz
•
11th Grade
