
Electron Configuration Review
Chemistry
10th - 12th Grade
NGSS covered
Used 10+ times

AI Actions
Add similar questions
Adjust reading levels
Convert to real-world scenario
Translate activity
More...
Content View
Student View
27 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
This "rule" of Quantum Chemistry states that it is impossible to know both the position and velocity of an electron at the same time.
2.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
Which shape represents the shape of an "s" orbital?
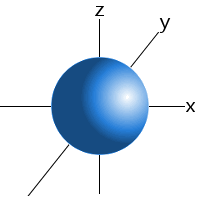
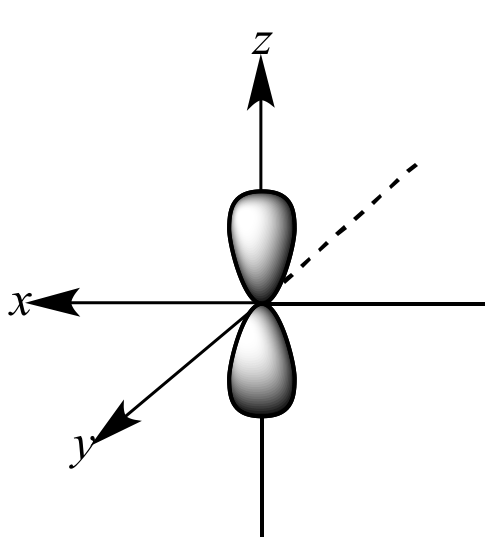
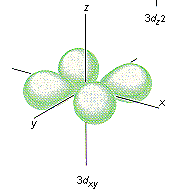
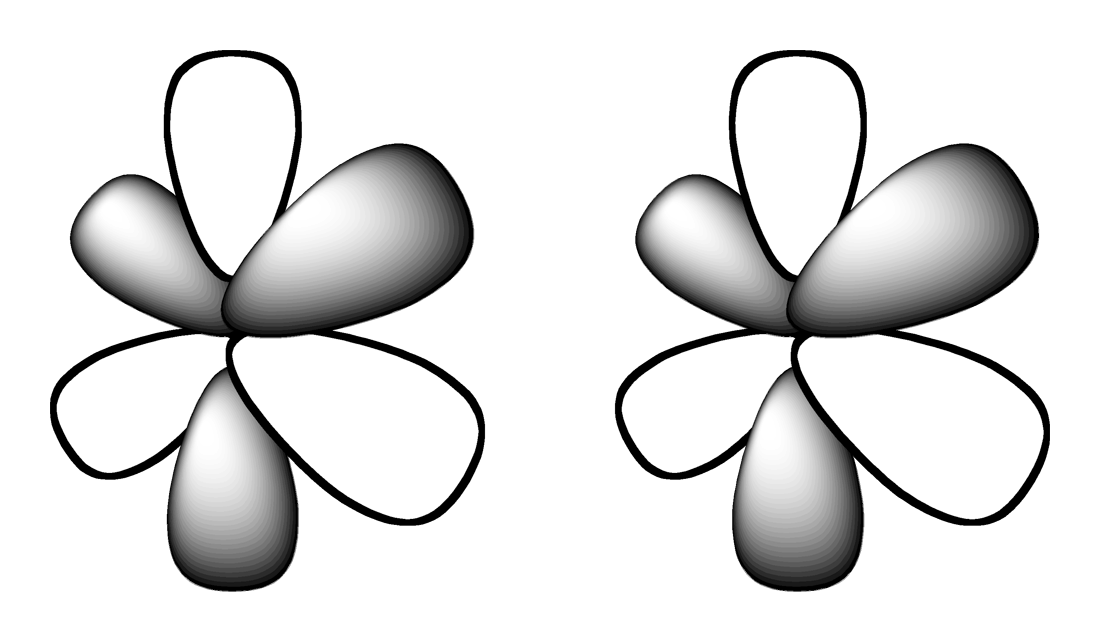
Tags
NGSS.HS-PS1-1
3.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
Which shape represents the shape of an "p" orbital?



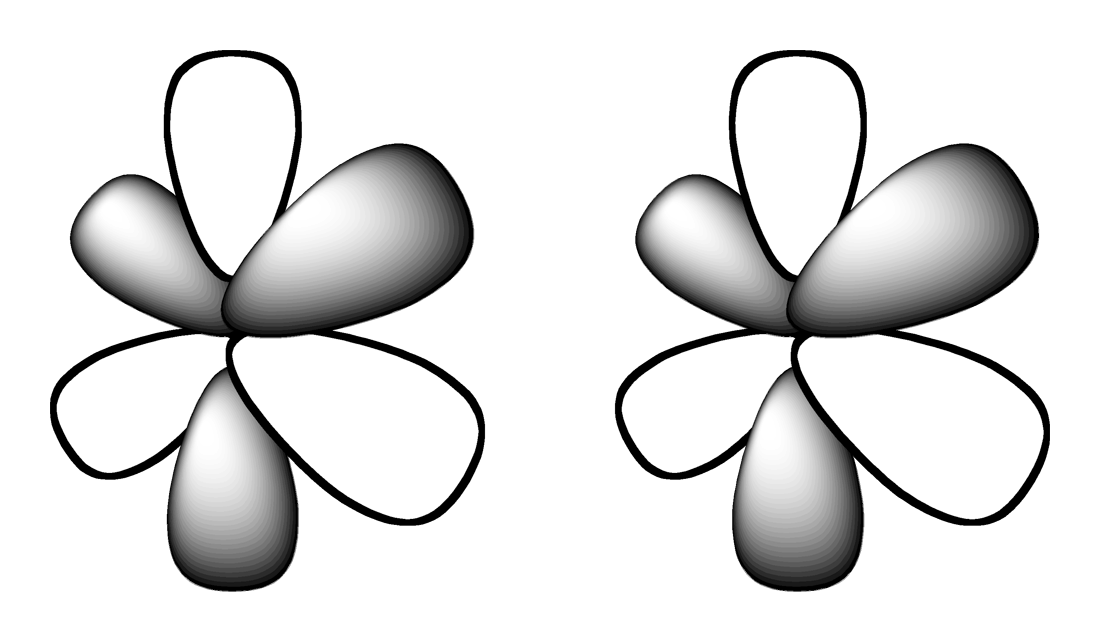
4.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
Which shape represents the shape of an "d" orbital?



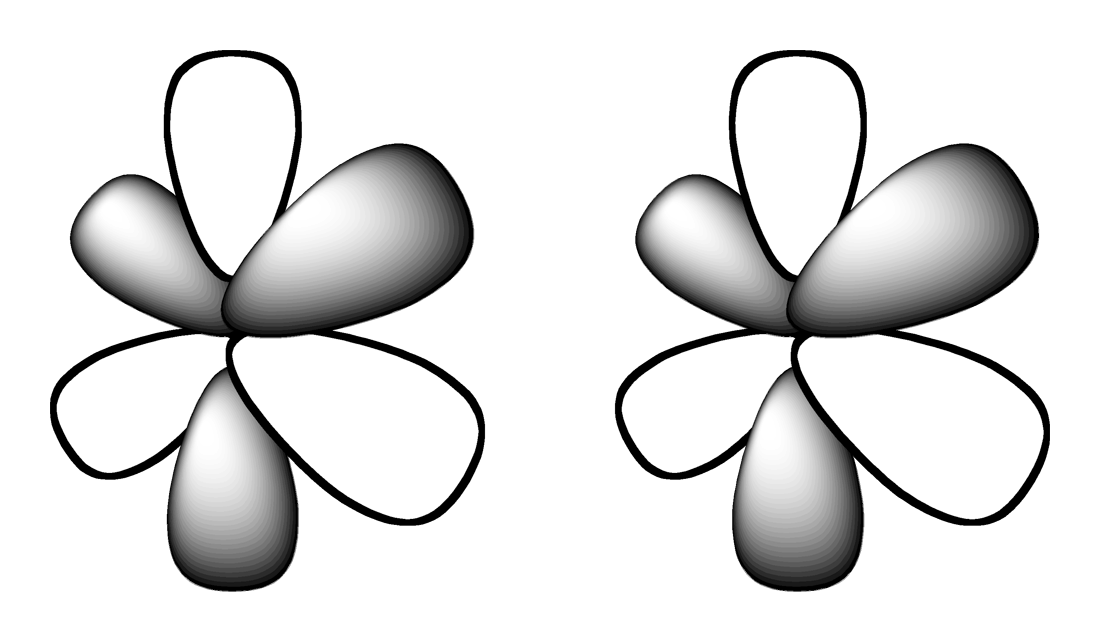
5.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
How many electrons can a single orbital hold?
1
2
3
5
6.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt

How many electrons can the p sublevel hold?
Tags
NGSS.HS-PS1-1
7.
MULTIPLE SELECT QUESTION
5 mins • 1 pt

Which of the following Aufbau diagrams are incorrect? Check all that apply.
A
B
C
D
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

22 questions
Lewis Structure of Elements
Quiz
•
10th - 12th Grade

22 questions
Real Gases
Quiz
•
9th - 12th Grade

23 questions
THÀNH PHẦN NGUYÊN TỬ BÀI TẬP TRẮC NGHIỆM NHIỀU LỰA CHỌN
Quiz
•
10th Grade

22 questions
TEORÍA CUÁNTICA Y TABLA PERIÓDICA
Quiz
•
10th Grade

22 questions
Lewis Structure
Quiz
•
10th - 12th Grade

22 questions
Electron Configuration
Quiz
•
10th - 12th Grade

22 questions
Ionic and Covalent Bonds
Quiz
•
9th Grade - University

22 questions
Moles to Moles Ratio
Quiz
•
9th - 12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

54 questions
Analyzing Line Graphs & Tables
Quiz
•
4th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

10 questions
Exploring Stoichiometry Concepts
Interactive video
•
6th - 10th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

20 questions
Practice: E-Con, Orbital Notation, Noble Gas Notation
Quiz
•
10th Grade

20 questions
Covalent Bonding
Quiz
•
10th Grade

10 questions
Periodic Table Families and Groups
Quiz
•
10th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade

22 questions
Solubility Curve Practice
Quiz
•
10th Grade


