
Acid Base Strength
Authored by Charles Martinez
Chemistry
11th - 12th Grade
NGSS covered

AI Actions
Add similar questions
Adjust reading levels
Convert to real-world scenario
Translate activity
More...
Content View
Student View
20 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt

Which of the following best approximates the Ka value for this weak acid?
1 x 10–3
1 x 10–4
1 x 10–5
1 x 10–6
2.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which of the following best represents a solution of H2SO4 in water?
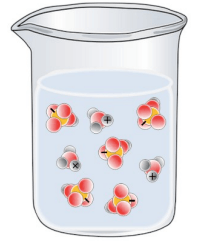
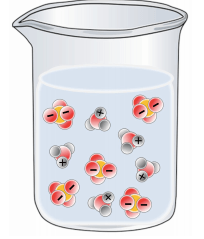
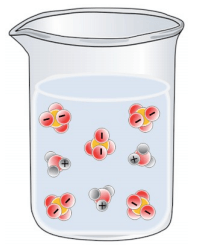
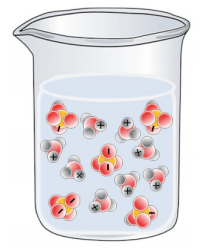
Tags
NGSS.HS-PS1-2
3.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
HSO4¯ + H2O ↔ H3O+ + SO4 2¯ In the equilibrium represented above, the species that act as bases include which of the following?
HSO4-
H2O
SO4 2¯
H2O and SO4 2¯
4.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Given the equation:
H2SO4 + H2O ↔ H3O+ + HSO4- 1
What is the conjugate acid?
H2SO4
H2O
H3O+
HSO4- 1
5.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which compound is the conjugate base?
HCO3- + HCl ==> H2CO3 + Cl-
Which compound is the conjugate base?
HCO3-
HCl
H2CO3
Cl-
6.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt
Which of the following is transferred between a conjugate acid-base pair?
an electron
a proton
a neutron
a OH- ion
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When a hydrogen ion is lost from an acid, the resulting particle is called its conjugate base. Which of the following particles would be the conjugate base for the acid HSO4–?
H2SO4
SO42–
SO4–
HSO4
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

15 questions
arrhenuis acid
Quiz
•
11th - 12th Grade

17 questions
Buffer solutions
Quiz
•
12th Grade - University

22 questions
Naming acids and bases
Quiz
•
10th - 12th Grade

19 questions
Kwasy karboksylowe
Quiz
•
8th Grade - Professio...

20 questions
soal pts kimia kelas xii
Quiz
•
12th Grade

22 questions
U4 - Physical vs. Chemical - Zeph
Quiz
•
9th - 12th Grade

25 questions
Rates/Rate law/Mechanisms
Quiz
•
11th - 12th Grade

18 questions
Single replacement Pr5,8
Quiz
•
9th - 12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

54 questions
Analyzing Line Graphs & Tables
Quiz
•
4th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade

5 questions
Mole Conversions Made Easy
Interactive video
•
11th Grade

10 questions
16. Limiting Reagent/% Yield Practice
Quiz
•
11th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

10 questions
PERIODIC TRENDS
Quiz
•
11th Grade

14 questions
Reaction Types, Balancing, and Predicting Products
Quiz
•
9th - 12th Grade

8 questions
Empirical and Molecular Formulas
Lesson
•
9th - 12th Grade