AP Chemistry Unit 8 Acids and Bases
Quiz
•
Chemistry
•
11th - 12th Grade
•
Medium
+1
Standards-aligned

Charles Martinez
Used 1+ times
FREE Resource
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt

A buffer solution is prepared by mixing equal volumes of 0.50 M weak acid with 1.0 M of its conjugate base. Based on the data given in the table above, which of the following pairs of chemical solutions should be used to prepare the buffer solution so that the pH will be between 4 and 7?
CH3COOH and NH3
CH3COOH and CH3COONa
H2CO3 and NH3
H2CO3 and Na2CO3
2.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt
Ascorbic acid, H2C6H6O6(s), is a diprotic acid with K1 = 7.9 × 10–5 and K2 = 1.6 × 10–12. In a 0.005 M aqueous solution of ascorbic acid, which of the following species is present in the lowest concentration?
H3O+(aq)
H2C6H6O6(aq)
HC6H6O6-(aq)
C6H6O62-(aq)
Tags
NGSS.HS-PS1-2
3.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt
In the titration of a weak acid of unknown concentration with a standard solution of a strong base, a pH meter was used to follow the progress of the titration. Which of the following is true for this experiment?
The [H+] at the equivalence point equals the ionization constant of the acid.
The pH at the equivalence point depends on the indicator used.
The graph of pH versus volume of base added rises gradually at first and then much more rapidly.
The graph of pH versus volume of base added shows no sharp rise.
4.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt
Which of the following is the correct equilibrium expression for the hydrolysis of CO3 2– ?
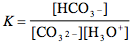
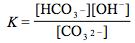
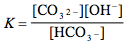
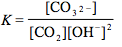
Tags
NGSS.HS-PS1-6
5.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt

Which of the following best approximates the Ka value for this weak acid?
1 x 10–3
1 x 10–4
1 x 10–5
1 x 10–6
6.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt

Which point on the titration curve corresponds to the point at which the moles of the added strong base are equal to the moles of the weak acid initially present?
Q
R
S
T
7.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt

At point P in the titration, which of the following species has the highest concentration?
HA
A–
H3O+
OH–
Create a free account and access millions of resources
Similar Resources on Wayground

12 questions
C6: ACID,BASE & SALT (F4)
Quiz
•
11th Grade

17 questions
pH scale and calculations
Quiz
•
9th - 12th Grade

15 questions
Identifying a Buffer Solution
Quiz
•
12th Grade

10 questions
AP Chemistry Buffers
Quiz
•
10th - 12th Grade

12 questions
Acid and Bases Revision
Quiz
•
11th Grade

12 questions
Acids/Bases & Neutralization Reactions
Quiz
•
9th - 12th Grade

10 questions
ATS KS3 Chemistry: Chemical Compounds
Quiz
•
7th - 12th Grade

10 questions
Acid-Base
Quiz
•
11th - 12th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Metric Conversions
Quiz
•
11th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

22 questions
SCIENCE LAB EQUIPMENT
Quiz
•
5th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade

18 questions
Classifying Matter Particle Diagrams
Quiz
•
11th - 12th Grade

19 questions
U2 Protons Neutrons and Electrons
Quiz
•
11th Grade