Chemical Bonding and Reactions
Quiz
•
Chemistry
•
9th Grade
•
Medium
Standards-aligned

Charles Martinez
Used 1+ times
FREE Resource
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Typically, atoms are more stable when they are
bonded together
apart from each other
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Why do atoms bond?
They typically don't bond
To add or take away energy levels
To have a full valance shell.
To have a full inner shell
Tags
NGSS.HS-PS1-2
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Ionic bonding is between a
nonmetal and nonmetal
metal and nonmetal
metal and metal
Depends on the situation
4.
MULTIPLE CHOICE QUESTION
20 sec • 1 pt
Covalent bonding is between a
nonmetal and nonmetal
metal and nonmetal
metal and metal
It depends on the situation
Tags
NGSS.HS-PS1-1
5.
MULTIPLE CHOICE QUESTION
20 sec • 1 pt

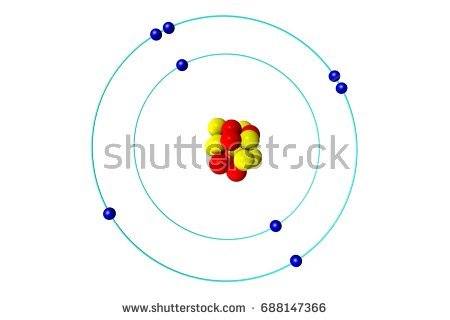
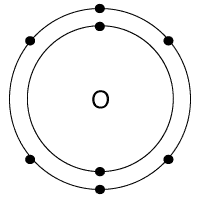
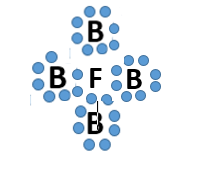
Which bond does this picture best represent?
Metallic bond
ionic bond
covalent bond
James Bond
Tags
NGSS.HS-PS1-1
6.
MULTIPLE CHOICE QUESTION
20 sec • 1 pt
What do positive ions tend to do?
lose electrons
gain electrons
lose protons
gain protons
7.
MULTIPLE CHOICE QUESTION
20 sec • 1 pt
What happens when magnesium loses 2 electrons?
It stabilizes to a net charge of 0
It turns into an atom
It becomes negatively charged
It becomes positively charged
Tags
NGSS.HS-PS1-2
Create a free account and access millions of resources
Similar Resources on Wayground

18 questions
Chemical Bonding
Quiz
•
6th - 10th Grade

20 questions
Ionic vs Covalent
Quiz
•
9th - 12th Grade

10 questions
Bonding and Lewis Dot Structures
Quiz
•
9th - 12th Grade

15 questions
Bond Formation
Quiz
•
9th Grade

15 questions
Chemical Bonds and Reactions
Quiz
•
9th Grade

15 questions
Bond
Quiz
•
9th Grade

15 questions
Chemical Bonding
Quiz
•
9th Grade

15 questions
Intro to Chemical Bonding
Quiz
•
9th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

22 questions
SCIENCE LAB EQUIPMENT
Quiz
•
5th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade

10 questions
Exploring Ionic Bonding: Definitions and Examples
Interactive video
•
6th - 10th Grade

18 questions
Crash Course: Nuclear Chemistry
Interactive video
•
9th - 12th Grade

15 questions
Heating & Cooling Curves Review
Quiz
•
9th - 12th Grade

25 questions
Tea Area Homecoming - 2025
Quiz
•
9th Grade - University