Lewis Structure VSEPR
Quiz
•
Chemistry
•
9th - 12th Grade
•
Practice Problem
•
Hard
+1
Standards-aligned

Charles Martinez
FREE Resource
Enhance your content in a minute
20 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt

CO2 has polar bonds but is a NON POLAR molecule. Why?
it has an asymmetrical shape
dipole moment between C and O atoms cancel
there is net dipole moment between C and O atoms in molecule
bond polarity does not exist between C+ and O- atoms
2.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt
Does the following reference Polar, Nonpolar, or both:
"electronegativity values are the same"?
Polar
Nonpolar
Both
3.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt

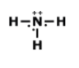
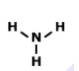
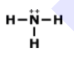
Is this molecule polar or nonpolar?
polar
nonpolar
4.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt
According to VSEPR, molecules adjust their shapes to keep which of the following as far away as possible?
5.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt
In a polar bond, the more electronegative element will assume a partial ________ charge.
Tags
NGSS.HS-PS1-1
6.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt
Type of bond between Br & Br
Ionic
Polar Covalent
Nonpolar Covalent
7.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt
Which molecule contains bonds with a GREATER polarity or which molecule is more polar?
Tags
NGSS.HS-PS1-1
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

20 questions
Molecular Geo, Polarity and IMFs
Quiz
•
9th - 12th Grade

15 questions
5.8 (4) Quiz: VSEPR
Quiz
•
10th - 12th Grade

20 questions
Acids and Bases
Quiz
•
9th Grade - University

20 questions
Acids, bases and salts test
Quiz
•
9th Grade

17 questions
Polymer Part 1
Quiz
•
12th Grade

15 questions
Grade 12 - Equilibria
Quiz
•
10th - 12th Grade

15 questions
Structural Formulas and VSEPR
Quiz
•
10th - 12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
Energy Transformations
Quiz
•
9th - 12th Grade

20 questions
Periodic Table & Trends
Quiz
•
10th Grade

17 questions
Reaction Rates
Quiz
•
11th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

24 questions
Identifying Types of Chemical Reactions
Quiz
•
10th - 12th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade