Lewis Structures with VSEPR
Quiz
•
Science
•
11th Grade
•
Practice Problem
•
Medium
Standards-aligned

Lisa Thompson
Used 3+ times
FREE Resource
Enhance your content in a minute
25 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
According to VSEPR, molecules adjust their shapes to keep which of the following as far away as possible?
Pairs of valence electrons
Inner shell electrons
Mobile Electrons
Electrons closest to the nucleus
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
How many electrons are shared in each bonding line?
1
2
3
4
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

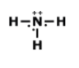
How many total valence electrons are participating in bonding in the molecule above?
8
4
2
3
Tags
NGSS.HS-PS1-2
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
According to VSEPR theory, what is the molecular geometry of a molecule with a central atom that has two bonding pairs and two lone pairs of electrons?
Linear
Bent
Tetrahedral
Trigonal Planar
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Which of the following is an acceptable Lewis structure for CH3Cl?
Option A
Option B
Option C
Option D
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Which of the following is the correct Lewis structure for the compound PBr3?
structure A
structure B
structure C
structure D
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which of the following molecules has a trigonal pyramidal shape according to VSEPR theory?
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

20 questions
EARTH SCIE (SOLAR SYSTEM)
Quiz
•
11th Grade

20 questions
Challenge
Quiz
•
9th - 12th Grade

20 questions
3.4 Exploring Metamorphic Rocks and Their Formation
Quiz
•
11th Grade

20 questions
Science Revision - Natural Resources
Quiz
•
5th Grade - University

20 questions
Seedlings and Transplanting
Quiz
•
10th - 12th Grade

20 questions
Science Week Brain Break Activity
Quiz
•
7th - 12th Grade

20 questions
NS: Features of Earth Quiz 1_sk
Quiz
•
4th Grade - University

20 questions
machines, our superpowers!
Quiz
•
4th Grade - University
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Science

14 questions
Natural Selection and Adaptation
Lesson
•
9th - 12th Grade

38 questions
Water Cycle and Groundwater
Quiz
•
9th - 12th Grade

12 questions
Cell Organelles and Their Functions
Lesson
•
9th - 12th Grade

12 questions
Homeostasis: Positive and Negative Feedback Loops
Interactive video
•
11th Grade

13 questions
Amoeba Sisters: Biomolecules
Interactive video
•
9th - 12th Grade

60 questions
2025 Genetics Test Study Guide
Quiz
•
9th - 12th Grade

10 questions
Understand Cell Functions and Organelles
Quiz
•
9th - 12th Grade

16 questions
Explore Cell Cycle Stages and Mitosis
Quiz
•
9th - 12th Grade