
Rate of Reactions
Quiz
•
Science
•
9th Grade
•
Medium
Standards-aligned

Lisa Thompson
Used 2+ times
FREE Resource
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
What is the meaning of the rate of reaction?
Decrease in amount of product
Decrease in amount of product against time
Increase in amount of products against time
Increase in amount of reactants against time
Tags
NGSS.HS-PS1-5
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
The rate of reaction for the decomposition of hydrogen peroxide decreases with time because
product of reaction decreases
temperature of hydrogen peroxide decreases
volume of hydrogen peroxide decreases
concentration of hydrogen peroxide decreases
Tags
NGSS.HS-PS1-5
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Products will form faster if____________
the particle size of the reactants are larger.
temperature is decreased.
concentration of the reactants are increased.
the reaction is not stirred.
Tags
NGSS.HS-PS1-5
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
The collision theory states that atoms, ions, and molecules must collide in order to react.
True
False
Tags
NGSS.HS-PS1-5
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
A catalyst works by
changing the order of the reaction
increasing the temperature
lowering the activation energy
making the activated complex
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
The following equation represents a chemical equation.
CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
Which graph shows the correct change in mass of reactant used in excess against time?


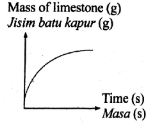
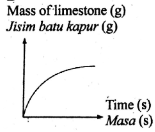
Tags
NGSS.HS-PS1-7
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which of the following explains the meaning of effective collision?
The collision where its energy is less than the activation energy
The collision that has a low energy
The collision which takes place before a reaction
The collision that causes a reaction
Tags
NGSS.HS-PS1-5
Create a free account and access millions of resources
Similar Resources on Wayground

20 questions
TEST REVIEW- Chemical Reactions
Quiz
•
6th Grade - University

16 questions
Balancing Reactions
Quiz
•
9th - 11th Grade

14 questions
homogenous and heterogenous catalysts
Quiz
•
10th - 12th Grade

15 questions
Reaction Rate
Quiz
•
11th Grade - University

15 questions
Kinetics AP Chemistry
Quiz
•
12th Grade - University

14 questions
Chemical Reactions
Quiz
•
7th - 9th Grade

17 questions
Conservation of Mass in Reactions
Quiz
•
8th Grade - University

10 questions
Predicting Products of Synthesis and Combustion Reactions
Quiz
•
9th - 12th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Science

10 questions
Exploring the Scientific Method
Interactive video
•
6th - 10th Grade

10 questions
Exploring Chemical and Physical Changes
Interactive video
•
6th - 10th Grade

17 questions
Enzymes
Quiz
•
9th Grade

10 questions
Exploring the Basics of Density
Interactive video
•
6th - 10th Grade

25 questions
Life Science Unit 1 Review
Quiz
•
7th - 9th Grade

10 questions
Kinetic and Potential Energy Explained
Interactive video
•
6th - 10th Grade

10 questions
The Evolution of Atomic Theory
Interactive video
•
6th - 10th Grade

10 questions
Exploring Biomes and Ecosystems for Kids
Interactive video
•
6th - 10th Grade
