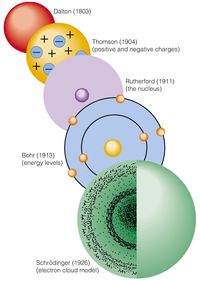
History of Atomic Models
Quiz
•
Science
•
8th Grade
•
Practice Problem
•
Medium
Standards-aligned

Lisa Thompson
Used 1+ times
FREE Resource
Enhance your content in a minute
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Who proposed that electrons move around the nucleus in circular orbits?
Dalton
Thomson
Rutherford
Bohr
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

One important difference between Bohr's model and the modern on is that electrons in the modern model are
moving in fixed orbits around the nucleus
moving in an electron cloud
moving in a random motion around the nucleus
not moving
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Who's model does this picture represent?
Dalton: 1803-1808
Thomson 1897
Rutherford 1911
Bohr 1913
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Who's model does this picture represent?
Dalton 1803-1808
Rutherford 1911
Thomson 1897
modern atomic model 1920 to the present
Tags
NGSS.MS-PS1-1
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Who's model does this represent?
Dalton 1903-1808
Rutherford 1911
Thomson 1897
Bohr 1913
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Who's model does this picture represent?
Dalton 1803-1808
Rutherford 1911
Bohr 1913
modern model 1920 to the present
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Who believed that electrons were scattered amongst positively charged material.
JJ Thomson
Ernest Rutherford
John Dalton
Niels Bohr
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

20 questions
QUIZ - PART 1
Quiz
•
8th Grade - University

15 questions
Scitech Quest
Quiz
•
8th Grade

20 questions
Sistem Pernapasan
Quiz
•
8th Grade

18 questions
8.5A & 8.5B Review
Quiz
•
8th Grade

20 questions
Year 9 Science | Inside the atom
Quiz
•
9th Grade

19 questions
3rd Mid-Quarter Exam in Science 9
Quiz
•
9th - 12th Grade

20 questions
Historical Timeline of Atom
Quiz
•
11th Grade

16 questions
End 1st 9wk Test Corrections
Quiz
•
8th Grade
Popular Resources on Wayground

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
4:3 Model Multiplication of Decimals by Whole Numbers
Quiz
•
5th Grade

10 questions
The Best Christmas Pageant Ever Chapters 1 & 2
Quiz
•
4th Grade

12 questions
Unit 4 Review Day
Quiz
•
3rd Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

15 questions
Solving Equations with Variables on Both Sides Review
Quiz
•
8th Grade
Discover more resources for Science

20 questions
Convection, Conduction, and Radiation
Quiz
•
6th - 8th Grade

10 questions
Exploring Heat Transfer: Conduction, Convection, and Radiation
Interactive video
•
6th - 8th Grade

10 questions
Exploring the Energy Cycle: Photosynthesis and Cellular Respiration
Interactive video
•
6th - 10th Grade

12 questions
Amoeba Sisters: Natural Selection
Interactive video
•
8th Grade

23 questions
Newton's 3 Laws of Motion
Quiz
•
8th Grade

11 questions
Force and Motion
Lesson
•
6th - 8th Grade

22 questions
Modeling Magnetic Force
Lesson
•
8th Grade

21 questions
Punnett Squares
Quiz
•
7th - 9th Grade
