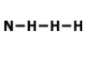Lewis Structures with VSEPR
Quiz
•
Science
•
11th Grade
•
Practice Problem
•
Easy
Standards-aligned

Lisa Thompson
Used 1+ times
FREE Resource
Enhance your content in a minute
25 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
According to VSEPR, molecules adjust their shapes to keep which of the following as far away as possible?
Pairs of valence electrons
Inner shell electrons
Mobile Electrons
Electrons closest to the nucleus
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
How many electrons are shared in each bonding line?
1
2
3
4
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

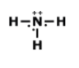
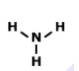
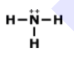
How many total valence electrons are participating in bonding in the molecule above?
8
4
2
3
Tags
NGSS.HS-PS1-2
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
According to VSEPR theory, what is the molecular geometry of a molecule with a central atom that has two bonding pairs and two lone pairs of electrons?
Linear
Bent
Tetrahedral
Trigonal Planar
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Which of the following is an acceptable Lewis structure for CH3Cl?
Option A
Option B
Option C
Option D
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Which of the following is the correct Lewis structure for the compound PBr3?
structure A
structure B
structure C
structure D
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which of the following molecules has a trigonal pyramidal shape according to VSEPR theory?
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

20 questions
PND1000 Midterm 2024
Quiz
•
University

20 questions
Suallarla Kosmosa 2
Quiz
•
University

20 questions
Gathering agistany
Quiz
•
University

20 questions
KESELAMATAN DAN PENGURUSAN MAKMAL
Quiz
•
University

22 questions
Midterm Review
Quiz
•
University

20 questions
3rd Summative Examination in Physical Science 11
Quiz
•
12th Grade

20 questions
GEOLOGIC PROCESS THAT SHAPES THE EARTH
Quiz
•
11th Grade

20 questions
Quiz #3
Quiz
•
11th Grade
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade
Discover more resources for Science

70 questions
Advanced Biology Semester Review
Quiz
•
9th - 12th Grade

19 questions
ionic vs covalent
Quiz
•
9th - 12th Grade

20 questions
Fall Final Review 2
Quiz
•
11th Grade

19 questions
Thermal Energy and Kinetic Theory
Quiz
•
9th - 12th Grade

18 questions
Unit 6: RNA & Protein Vocab
Quiz
•
9th - 12th Grade

12 questions
Fall Final Review 1
Quiz
•
11th Grade