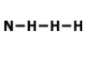Drawing Lewis Dot Structures
Quiz
•
Science
•
10th Grade
•
Practice Problem
•
Hard
Standards-aligned

Lisa Thompson
Used 1+ times
FREE Resource
Enhance your content in a minute
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
How many electrons should Nitrogen have around its Lewis dot model?
1
3
4
5
2.
REORDER QUESTION
1 min • 1 pt
Reorder the following steps for drawing lewis structures
Add remaining electrons as Lone pairs (terminal atoms first).
Check each atom has the same number of electrons as it started with (formal charge)
Place the lower Electronegative atom in the centre and draw in bonds
The Valence electrons
charge - add/subtract if there is a charge
If any atoms dont have an Octet, use electrons to create double bonds.
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

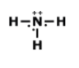
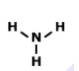
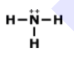
Which of the following is the correct Lewis structure for the compound PBr3?
structure A
structure B
structure C
structure D
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
4.
DRAW QUESTION
1 min • 1 pt
Draw the lewis structure for CH3NH2

Tags
NGSS.HS-PS1-1
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

This could be the dot diagram of
Mg
Cl
C
O
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
6.
MULTIPLE CHOICE QUESTION
1 min • 12 pts
Each line in a Lewis Structure represents __________ electron(s).
3
2
1
4
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which Lewis Dot Model drawing correctly shows an O2 molecule?




Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

10 questions
Honey Bees
Quiz
•
9th - 12th Grade

10 questions
HANDWASHING FACT VS MYTH
Quiz
•
9th - 12th Grade

10 questions
Brain & Nervous System
Quiz
•
9th - 12th Grade

10 questions
CONVERGENT BOUNDARY
Quiz
•
10th Grade

20 questions
UH IPA 7 KLASIFIKASI ZAT & PERUBAHANNYA
Quiz
•
10th Grade

15 questions
Y9 chemistry quiz on air and water
Quiz
•
7th - 10th Grade

10 questions
Our Future
Quiz
•
KG - 12th Grade

13 questions
Atomic Structure
Quiz
•
7th Grade - University
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Science

10 questions
Exploring the Layers of the Earth
Interactive video
•
6th - 10th Grade

14 questions
Natural Selection and Adaptation
Lesson
•
9th - 12th Grade

10 questions
Exploring the Ring of Fire: Volcanoes and Earthquakes
Interactive video
•
6th - 10th Grade

38 questions
Water Cycle and Groundwater
Quiz
•
9th - 12th Grade

10 questions
Exploring Spring and Neap Tides
Interactive video
•
6th - 10th Grade

12 questions
Cell Organelles and Their Functions
Lesson
•
9th - 12th Grade

22 questions
Earth's Spheres and Interactions Quiz Reveiw
Quiz
•
8th - 10th Grade

16 questions
Air Masses & Fronts
Lesson
•
8th - 10th Grade