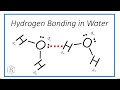
Hydrogen Bonds and Water Properties
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Olivia Brooks
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the shape of a water molecule due to the presence of lone pairs?
Tetrahedral
Trigonal planar
Bent
Linear
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why do water molecules have a bent shape?
Because of the equal sharing of electrons
Due to the absence of lone pairs
Due to the presence of lone pairs pushing hydrogen atoms
Because of the linear arrangement of atoms
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What causes the partial negative charge on the oxygen atom in a water molecule?
Absence of lone pairs
Equal sharing of electrons
Oxygen's higher electronegativity
Hydrogen's higher electronegativity
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is water considered a polar molecule?
Because of the unequal sharing of electrons
Due to the equal sharing of electrons
Because it has a symmetrical shape
Due to the absence of partial charges
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does a hydrogen bond form between two water molecules?
Between the nuclei of two molecules
Between a lone pair on one molecule and a hydrogen on another
Between two oxygen atoms
Between two hydrogen atoms
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of lone pairs in hydrogen bonding between water molecules?
They attract other lone pairs
They repel other water molecules
They form bonds with hydrogen atoms of other molecules
They do not participate in bonding
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many hydrogen bonds can a single water molecule potentially form?
Two
Three
Four
Five
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the maximum number of hydrogen bonds a water molecule can form with other water molecules?
Three
One
Two
Four
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

11 questions
NEASC Extended Advisory
Lesson
•
9th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

21 questions
Isotopes and Ions
Quiz
•
9th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade