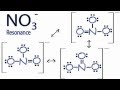
Resonance Structures of NO3-
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Ethan Morris
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in analyzing resonance for NO3-?
Calculating molecular weight
Measuring bond angles
Drawing a Lewis structure
Identifying the number of protons
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many different ways can the double bond be placed in the Lewis structure of NO3-?
Two ways
One way
Three ways
Four ways
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What do the resonance structures of NO3- help us understand?
The molecular weight
The distribution of electrons
The boiling point
The color of the compound
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the real world, how is NO3- represented?
As a pair of structures
As an average of three structures
As a dynamic structure
As a single structure
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the nature of the bonds in the real-world representation of NO3-?
Double bonds only
Single bonds only
Triple bonds
One and a third bonds
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why do we draw resonance structures?
To predict color
To understand electron distribution
To calculate molecular weight
To determine boiling point
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a limitation of Lewis structures?
They are three-dimensional
They are too colorful
They cannot accurately depict electron distribution
They are too complex
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does experimental data show about the bonds in NO3-?
They are all triple bonds
They are all single bonds
They are all double bonds
They are one and a third bonds
Similar Resources on Wayground

11 questions
Nitrate Ion Properties and Behavior
Interactive video
•
9th - 10th Grade

11 questions
Valence Electrons and Lewis Structures
Interactive video
•
9th - 10th Grade

11 questions
Lewis Structures and Valence Electrons
Interactive video
•
9th - 10th Grade

11 questions
Lewis Structures and Formal Charges
Interactive video
•
9th - 10th Grade

11 questions
Phosphate Ion Lewis Structure Concepts
Interactive video
•
9th - 10th Grade

11 questions
Nitrogen Dioxide Molecular Geometry Concepts
Interactive video
•
9th - 10th Grade

11 questions
Molecular Geometry and Lewis Structures
Interactive video
•
9th - 10th Grade

10 questions
Lewis Structures and Formal Charges
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade