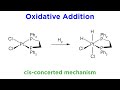
Oxidative Addition in Coordination Chemistry
Interactive Video
•
Chemistry
•
11th - 12th Grade
•
Hard
Sophia Harris
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a key requirement for a metal to undergo oxidative addition?
The metal must have a stable oxidation state.
The metal must be in a gaseous state.
The metal must be a noble gas.
The metal must be radioactive.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a concerted oxidative addition, how do the ligands typically arrange themselves?
In a linear fashion
Randomly
Cis to each other
Trans to each other
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which type of bond is most commonly involved in concerted oxidative addition?
Nonpolar bonds
Ionic bonds
Polar bonds
Metallic bonds
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What role does the dz2 orbital play in oxidative addition involving an iridium complex?
It donates electron density to the ligands.
It remains inactive during the reaction.
It projects lobes along the axis with open coordination sites.
It forms a covalent bond with the ligands.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In stepwise oxidative addition, which part of the polar molecule does the metal interact with first?
The less electronegative end
The center of the molecule
The more electronegative end
The entire molecule at once
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a characteristic of the intermediate formed in stepwise oxidative addition?
It is always neutral.
It has a lower oxidation state than the original metal.
It is highly unstable and decomposes immediately.
It has a formal charge one greater than before.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which type of bond is more challenging to activate but is synthetically important?
C-H bonds
C-C bonds
N-H bonds
O-H bonds
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Popular Resources on Wayground

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

20 questions
Halloween Trivia
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

4 questions
Activity set 10/24
Lesson
•
6th - 8th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
How to Email your Teacher
Quiz
•
Professional Development

15 questions
Order of Operations
Quiz
•
5th Grade

30 questions
October: Math Fluency: Multiply and Divide
Quiz
•
7th Grade
Discover more resources for Chemistry

20 questions
States of Matter
Lesson
•
7th - 12th Grade

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

28 questions
Unit 4 Ionic Bonding/Nomenclature
Quiz
•
10th - 12th Grade

12 questions
Chemistry - Lewis Dot Structures
Quiz
•
10th - 12th Grade

16 questions
Bohr Models
Quiz
•
9th - 11th Grade

26 questions
Ionic Compounds: Naming and Formula Writing
Quiz
•
9th - 12th Grade

15 questions
Periodic Trends
Quiz
•
10th - 11th Grade