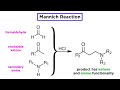
Mannich Reaction Concepts and Applications
Interactive Video
•
Chemistry
•
11th - 12th Grade
•
Hard
Mia Campbell
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Who invented the Mannich reaction?
Robert Bunsen
Albert Eschenmoser
Carl Mannich
Friedrich Wohler
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are the three components used in the original Mannich reaction?
Ketone, a tertiary amine, and water
Formaldehyde, a primary amine, and an ester
Aldehyde, primary amine, and a ketone
Formaldehyde, an enolizable ketone, and a secondary amine
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of the acid in the Mannich reaction mechanism?
To dehydrate the ketone
To catalyze the formation of the iminium ion
To reduce the formaldehyde
To oxidize the amine
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is an Eschenmoser salt?
A byproduct of the Mannich reaction
A catalyst for aldol reactions
A moisture-sensitive salt used in Mannich reactions
A type of ketone
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a key application of the Mannich reaction in methylenation?
Formation of a quaternary ammonium salt
Reduction of ketones
Oxidation of alcohols
Hydration of alkenes
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which compounds can react with Eschenmoser salts in Mannich-like reactions?
Only aldehydes
Only electron-poor aromatics
Only ketone enolates
Enolates from esters, nitriles, and nitro compounds
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a significant challenge when using aldehydes other than formaldehyde in the Mannich reaction?
Inability to form iminium ions
Formation of multiple products
Lack of reactivity
Formation of several diastereomers
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

8 questions
Halogen Compounds:Methods of Preparation
Interactive video
•
10th - 12th Grade

8 questions
Le Chatelier's Principle
Interactive video
•
11th Grade - University

8 questions
Magnetismo y electricidad
Interactive video
•
11th Grade - University

6 questions
Thermochemistry: Heat and Enthalpy
Interactive video
•
11th Grade - University

6 questions
Oxidation-Reduction Reactions
Interactive video
•
11th Grade - University

6 questions
Practice Problem: Gravimetric Analysis
Interactive video
•
11th Grade - University

11 questions
Molecular Orbital and Reaction Mechanisms
Interactive video
•
11th - 12th Grade

11 questions
Catalytic Hydrogenation Concepts and Challenges
Interactive video
•
11th - 12th Grade
Popular Resources on Wayground

20 questions
Halloween Trivia
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Order of Operations
Quiz
•
5th Grade

20 questions
Halloween
Quiz
•
5th Grade

16 questions
Halloween
Quiz
•
3rd Grade

12 questions
It's The Great Pumpkin Charlie Brown
Quiz
•
1st - 5th Grade

20 questions
Possessive Nouns
Quiz
•
5th Grade

10 questions
Halloween Traditions and Origins
Interactive video
•
5th - 10th Grade
Discover more resources for Chemistry

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade

55 questions
Unit 4: A Conflict of Cans Summative Review
Quiz
•
11th Grade

15 questions
Electron Configurations and Orbital Notation
Quiz
•
11th Grade

10 questions
Chemistry Halloween Quiz
Quiz
•
12th Grade

14 questions
PERIODIC TRENDS
Quiz
•
11th Grade

16 questions
Naming Ionic Compounds
Quiz
•
9th - 11th Grade

10 questions
Isotopes
Quiz
•
9th - 12th Grade

15 questions
Calculating Density
Quiz
•
11th Grade