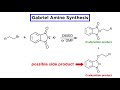
Gabriel Synthesis and Reaction Mechanisms
Interactive Video
•
Chemistry
•
11th - 12th Grade
•
Hard
Mia Campbell
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary reason for naming certain reactions after chemists?
To increase their popularity
To honor their contributions to chemistry
To differentiate them from other reactions
To make them easier to refer to
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What problem does the Gabriel synthesis primarily address?
Difficulty in synthesizing primary amines
Low yield of secondary amines
Complex mixtures of amines
Formation of quaternary ammonium salts
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the Gabriel synthesis, what role does phthalimide play?
Acts as a catalyst
Prevents the formation of alkenes
Serves as a doubly protected version of ammonia
Increases the reaction rate
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is hydrazine used in the deprotection step of the Gabriel synthesis?
It prevents side reactions
It enhances the reaction speed
It is a mild nucleophile
It is a strong acid
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a potential side reaction in the Gabriel synthesis?
Formation of secondary amines
Formation of quaternary ammonium salts
Formation of alkenes
Formation of O-alkylation product
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the main challenge in distinguishing between N-alkylation and O-alkylation products?
They have different boiling points
They react differently with acids
They have different solubilities
They have similar spectral characteristics
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a key advantage of using differentially protected ammonia surrogates in Gabriel synthesis?
They allow for the synthesis of secondary amines
They increase the reaction rate
They simplify the reaction mechanism
They prevent the formation of side products
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

11 questions
Stoichiometry and Chemical Reactions
Interactive video
•
10th - 12th Grade

11 questions
Understanding SN2 Reactions and Stereochemistry
Interactive video
•
10th - 12th Grade

11 questions
Understanding Pre-Equilibrium Approximation in Reaction Mechanisms
Interactive video
•
10th - 12th Grade

11 questions
Organic Chemistry Reaction Mechanisms
Interactive video
•
10th Grade - University

11 questions
CRISPR and Genetic Engineering Concepts
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

11 questions
NEASC Extended Advisory
Lesson
•
9th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade

34 questions
Covalent and Ionic Bonds Concepts
Quiz
•
9th - 12th Grade

12 questions
Unit 2 P #6 Electron configuration and Orbital diagrams
Quiz
•
10th - 12th Grade

20 questions
Binary Ionic Compounds (Group A Elements)
Quiz
•
11th Grade

18 questions
Ions
Quiz
•
9th - 12th Grade