Covalent Bonding and VSEPR Theory Insights
Interactive Video
•
Chemistry
•
6th - 10th Grade
•
Easy
Mia Campbell
Used 3+ times
FREE Resource
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary focus of the video tutorial?
Balancing chemical equations
Understanding molecular shapes and dot models
Learning about ionic bonds
Studying the periodic table
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does hydrogen have?
One
Three
Two
Four
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
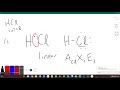
What is the total number of valence electrons in HCl?
Nine
Eight
Seven
Six
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a dot model, how are shared pairs of electrons represented?
As squares
As triangles
As lines
As circles
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the shape of a two-atom system according to the VSEPR method?
Linear
Trigonal planar
Bent
Tetrahedral
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which element is always the central atom if present in a molecule?
Fluorine
Hydrogen
Carbon
Oxygen
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the shape of CF4 according to the VSEPR method?
Bent
Tetrahedral
Trigonal planar
Linear
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Molecular Geometry Insights Through VSEPR Model
Interactive video
•
9th - 10th Grade

8 questions
Steric Number and Molecular Geometry
Interactive video
•
9th - 10th Grade

9 questions
Boron and Fluorine in BF3
Interactive video
•
9th - 10th Grade

11 questions
Understanding the Lewis Structure of NH3
Interactive video
•
7th - 10th Grade

11 questions
Molecular Geometry and Valence Electrons
Interactive video
•
6th - 12th Grade

8 questions
Molecular Geometry of CO2
Interactive video
•
9th - 10th Grade

11 questions
Molecular Geometry and Bonding Concepts
Interactive video
•
9th - 10th Grade

11 questions
Formaldehyde Molecular Structure Concepts
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

50 questions
Trivia 7/25
Quiz
•
12th Grade

11 questions
Standard Response Protocol
Quiz
•
6th - 8th Grade

11 questions
Negative Exponents
Quiz
•
7th - 8th Grade

12 questions
Exponent Expressions
Quiz
•
6th Grade

4 questions
Exit Ticket 7/29
Quiz
•
8th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade

20 questions
One Step Equations All Operations
Quiz
•
6th - 7th Grade

18 questions
"A Quilt of a Country"
Quiz
•
9th Grade
Discover more resources for Chemistry

11 questions
Standard Response Protocol
Quiz
•
6th - 8th Grade

11 questions
Negative Exponents
Quiz
•
7th - 8th Grade

12 questions
Exponent Expressions
Quiz
•
6th Grade

4 questions
Exit Ticket 7/29
Quiz
•
8th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade

20 questions
One Step Equations All Operations
Quiz
•
6th - 7th Grade

18 questions
"A Quilt of a Country"
Quiz
•
9th Grade

13 questions
Primary and Secondary Sources
Quiz
•
6th Grade