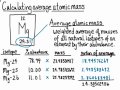
Calculating Average Atomic Mass and Isotope Abundance
Interactive Video
•
Chemistry
•
6th - 10th Grade
•
Easy
Sophia Harris
Used 1+ times
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the average atomic mass of magnesium as shown in the periodic table?
12.00
24.31
28.09
26.98
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the atomic number of magnesium?
11
13
12
10
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a weighted average in the context of atomic mass?
An average of the masses of all isotopes
An average of the masses of the most common isotopes
An average that takes into account the natural abundance of each isotope
An average that ignores the natural abundance of isotopes
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which isotope of magnesium is the most abundant?
Magnesium-26
Magnesium-25
Magnesium-27
Magnesium-24
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you calculate the average atomic mass of an element?
By adding the masses of all isotopes and dividing by the number of isotopes
By averaging the masses of the two most common isotopes
By multiplying the mass of each isotope by its fractional abundance and summing the results
By taking the mass of the most abundant isotope
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the mass number of the most common isotope of magnesium?
24
25
23
26
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why are atomic mass units not whole numbers?
Because of rounding errors
Because isotopes have different masses
Because electrons are included in the mass
Because protons and neutrons have slightly different masses
Create a free account and access millions of resources
Similar Resources on Wayground

6 questions
Peso atómico
Interactive video
•
4th - 9th Grade

8 questions
Understanding Hydrogen Isotopes and Atomic Number
Interactive video
•
6th - 8th Grade

11 questions
Exploring Isotopes and Atomic Mass Calculations
Interactive video
•
6th - 10th Grade

11 questions
Subatomic Particles and Atomic Structure
Interactive video
•
6th - 10th Grade

6 questions
Understanding Elements and Atomic Structure
Interactive video
•
7th - 10th Grade

11 questions
Exploring Atomic Structure and Isotopes
Interactive video
•
6th - 10th Grade

11 questions
Exploring Atoms to Ions: Part 3 Concepts
Interactive video
•
6th - 10th Grade

11 questions
Exploring Atomic Structure and the Periodic Table
Interactive video
•
6th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Appointment Passes Review
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
Grammar Review
Quiz
•
6th - 9th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

20 questions
Physical and Chemical Properties
Quiz
•
8th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

20 questions
States of Matter
Quiz
•
8th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade