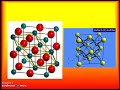
Exploring Ionic Bonds and Their Properties
Interactive Video
•
Mathematics
•
6th - 10th Grade
•
Practice Problem
•
Medium
Emma Peterson
Used 2+ times
FREE Resource
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are the two types of ions that form ionic compounds?
Neutrons and Electrons
Electrons and Protons
Protons and Neutrons
Anions and Cations
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why are ionic compounds electrically neutral overall?
Because they have equal numbers of protons and neutrons
Because they combine in combinations that balance each other out
Because they are composed of only one type of ion
Because they have no charged particles
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of bond is formed between a positive cation and a negative anion?
Covalent bond
Ionic bond
Hydrogen bond
Metallic bond
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the example of sodium chloride, what is the ratio of sodium ions to chloride ions?
1:1
3:1
1:2
2:1
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a formula unit in the context of ionic compounds?
The molecular weight of the compound
The total number of ions in a compound
The smallest whole number ratio of ions that makes the compound neutral
The volume of the compound
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is NOT a characteristic of ionic crystalline structures?
They have a repeating three-dimensional pattern
They have high melting points
They are formed by the attraction of oppositely charged ions
They are composed of separate individual particles
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the coordination number of sodium chloride?
4
6
8
10
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

54 questions
Analyzing Line Graphs & Tables
Quiz
•
4th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade
Discover more resources for Mathematics

22 questions
distributive property
Quiz
•
7th Grade

18 questions
Angle Relationships
Quiz
•
7th Grade

15 questions
Distributive Property & Review
Quiz
•
6th Grade

20 questions
Writing Algebraic Expressions
Quiz
•
6th Grade

20 questions
How Some Friendships Last — and Others Don’t Video Questions
Quiz
•
7th Grade

14 questions
finding slope from a graph
Quiz
•
8th Grade

20 questions
Ratios/Rates and Unit Rates
Quiz
•
6th Grade

18 questions
Handbook Refresher Quiz
Quiz
•
7th Grade