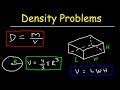
Solving Density Problems and Formulas
Interactive Video
•
Mathematics
•
6th - 10th Grade
•
Practice Problem
•
Hard
+1
Standards-aligned
Jackson Turner
FREE Resource
Standards-aligned
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the density of a rock with a mass of 50 g and a volume of 8.5 ml?
8.33 g/ml
5.88 g/ml
6.25 g/ml
7.14 g/ml
Tags
CCSS.HSG.MG.A.2
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If a metal bar has a density of 7.9 g/ml and a volume of 500 ml, what is its mass in grams?
4,000 g
3,500 g
4,500 g
3,950 g
Tags
CCSS.HSG.MG.A.2
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the volume of a rectangular metal bar with dimensions 6 cm by 8 cm by 9 cm?
432 cubic cm
450 cubic cm
480 cubic cm
400 cubic cm
Tags
CCSS.5.MD.C.5B
CCSS.6.G.A.2
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you convert grams per milliliter to kilograms per cubic meter?
Multiply by 1000
Divide by 1000
Multiply by 100
Divide by 100
Tags
CCSS.5.MD.A.1
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the density of a rock with a mass of 25 g and a volume of 3.1 ml?
9.15 g/ml
7.25 g/ml
8.06 g/ml
6.45 g/ml
Tags
CCSS.HSG.MG.A.2
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Will a spherical ball with a density of 2.29 g/ml sink or float in pure water?
Both
Neither
Float
Sink
Tags
CCSS.HSG.MG.A.2
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the new volume level in a graduated cylinder if an 8.5 karat diamond is added to 54.3 ml of water?
55.3 ml
54.8 ml
55.0 ml
54.5 ml
Tags
CCSS.HSG.MG.A.2
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Mathematics

20 questions
Exponents
Quiz
•
6th Grade

22 questions
distributive property
Quiz
•
7th Grade

15 questions
Distributive Property & Review
Quiz
•
6th Grade

20 questions
Writing Algebraic Expressions
Quiz
•
6th Grade

20 questions
Ratios/Rates and Unit Rates
Quiz
•
6th Grade

20 questions
Writing and Graphing Inequalities
Quiz
•
6th Grade

15 questions
Product of Powers Property A1 U7
Quiz
•
8th Grade

20 questions
Laws of Exponents
Quiz
•
8th Grade