Percent Yield and Percent Error Explained
Interactive Video
•
Mathematics
•
6th - 10th Grade
•
Practice Problem
•
Hard
Standards-aligned
Ethan Morris
FREE Resource
Standards-aligned
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the law that states mass can neither be created nor destroyed?
Law of Definite Proportions
Law of Multiple Proportions
Law of Conservation of Mass
Law of Constant Composition
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
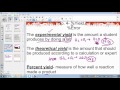
What is the term for the amount of product expected based on calculations?
Actual Yield
Practical Yield
Experimental Yield
Theoretical Yield
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If a student gets 34.2 grams of water instead of the expected 36 grams, what is the 34.2 grams called?
Theoretical Yield
Actual Yield
Experimental Yield
Predicted Yield
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you calculate percent yield?
Theoretical Yield divided by Experimental Yield times 100
Theoretical Yield minus Experimental Yield times 100
Experimental Yield divided by Theoretical Yield times 100
Experimental Yield minus Theoretical Yield times 100
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the example given, what is the percent yield if the experimental yield is 34.2 grams and the theoretical yield is 36 grams?
94.8%
95.5%
94.5%
95%
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does a percent yield of 100% indicate?
The reaction had some errors
The reaction was perfect with no errors
The reaction was overestimated
The reaction was incomplete
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the theoretical yield in the example problem where the expected amount is 125 grams?
125 grams
150 grams
92.5 grams
100 grams
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Exploring Percent Error and Percent Change
Interactive video
•
6th - 10th Grade

11 questions
Decimal, Percent, and Fraction Conversion Challenge
Interactive video
•
6th - 10th Grade

11 questions
Estimating Voter Support for Brown
Interactive video
•
7th - 10th Grade

11 questions
Fraction, Decimal, and Percent Conversion Challenge
Interactive video
•
6th - 10th Grade

11 questions
Exploring Experimental and Theoretical Probability
Interactive video
•
6th - 10th Grade

11 questions
Exploring the Volume of Cylinders
Interactive video
•
6th - 10th Grade

11 questions
Exploring Percent Change and Percent Error
Interactive video
•
6th - 10th Grade

11 questions
Mastering Percent Word Problems
Interactive video
•
6th - 10th Grade
Popular Resources on Wayground

7 questions
History of Valentine's Day
Interactive video
•
4th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

15 questions
Valentine's Day Trivia
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade
Discover more resources for Mathematics

20 questions
Writing Algebraic Expressions
Quiz
•
6th Grade

20 questions
Ratios/Rates and Unit Rates
Quiz
•
6th Grade

14 questions
Volume of rectangular prisms
Quiz
•
7th Grade

20 questions
Laws of Exponents
Quiz
•
8th Grade

20 questions
Graphing Inequalities on a Number Line
Quiz
•
6th - 9th Grade

20 questions
One Step Equations
Quiz
•
6th Grade

20 questions
Exponent Properties
Quiz
•
9th Grade

10 questions
Solving One Step Inequalities
Quiz
•
6th Grade