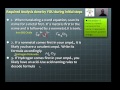
Mastering Chemical Equations: From Words to Balance
Interactive Video
•
Chemistry
•
9th - 12th Grade
•
Practice Problem
•
Easy
Liam Anderson
Used 1+ times
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What term describes a chemical equation expressed in words?
Skeleton equation
Balanced equation
Chemical notation
Word equation
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What symbol is used in chemical equations to indicate a reaction produces a product?
Plus (+)
Arrow (→)
Equals (=)
Asterisk (*)
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the state of matter notation for gases in chemical equations?
(s)
(aq)
(l)
(g)
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a balanced chemical equation, what must the number of atoms of each element in the reactants side be relative to the products side?
Not related
Equal to
Less than
Greater than
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the correct formula for water in chemical equations?
H2O
O2
H2
HO
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which method is suggested for balancing charges in ionic compounds?
Addition method
Subtraction method
Crisscross method
Division method
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does a Roman numeral in an ionic compound's name indicate?
The number of molecules
The charge on the nonmetal
The charge on the metal
The number of atoms
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

14 questions
General Technology Use Quiz
Quiz
•
8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

19 questions
Thanksgiving Trivia
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 4/5-Covalent Bonding/Nomenclature
Quiz
•
10th Grade

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

20 questions
Ions
Quiz
•
10th Grade

25 questions
VSPER Shape Quiz
Quiz
•
10th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade

14 questions
PERIODIC TRENDS
Quiz
•
11th Grade

61 questions
KAP Chemistry Covalent Test Review
Quiz
•
10th Grade

27 questions
Unit 4/5 Covalent Bonding/Nomenclature
Quiz
•
10th - 12th Grade