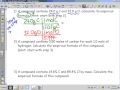
Moles and Stoichiometry: Empirical Formula Challenges
Interactive Video
•
Chemistry
•
9th - 12th Grade
•
Medium
Mia Campbell
Used 1+ times
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What unit is used to convert grams of an element to moles in the dimensional analysis?
Liters per mole
Mole ratio
Percentage by mass
Grams per mole
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the gram formula mass (gfm) used for in the conversion process?
To calculate the yield of a reaction
To convert grams to moles
To determine the compound's density
To convert moles to liters
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does dimensional analysis help you find in the context of empirical formulas?
The concentration of solutions
The energy change per mole
The moles of each element
The volume of reactants
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What do you need to calculate after finding the moles of each element to determine the empirical formula?
Molecular weight
Percentage composition
Lowest whole number ratio
Total mass of the compound
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If given moles directly in a problem, what is the next step to find the empirical formula?
Assume a 100 gram sample
Divide by the lowest mole number
Find the molecular formula
Convert to grams
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is the empirical formula written after determining the mole ratios?
As a balanced equation
Using molecular weights
With subscripts indicating mole ratios
As a percentage composition
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to divide by the lowest mole number when calculating empirical formulas?
To ensure the formula is as simple as possible
To balance the chemical equation
To calculate the reactant's purity
To determine the reaction rate
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Empirical Formulas and Molar Mass
Interactive video
•
9th - 10th Grade

11 questions
Calculating Gram Formula Mass and Composition
Interactive video
•
9th - 12th Grade

11 questions
Water of Hydration Lab Concepts
Interactive video
•
9th - 10th Grade

11 questions
Hydrates and Molar Mass Concepts
Interactive video
•
9th - 10th Grade

11 questions
Stoichiometry and Empirical Formulas Quiz
Interactive video
•
9th - 12th Grade

11 questions
Understanding Molecular Representations
Interactive video
•
9th - 12th Grade

11 questions
Empirical and Molecular Formulas
Interactive video
•
9th - 12th Grade

11 questions
Understanding Compounds and Formulas
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Appointment Passes Review
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
Grammar Review
Quiz
•
6th - 9th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade