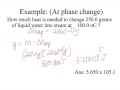
Thermal Energy and Phase Changes
Interactive Video
•
Chemistry, Science
•
9th - 12th Grade
•
Medium
Lucas Foster
Used 1+ times
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is NOT a phase change?
Condensation
Combustion
Melting
Freezing
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
During which part of the heating curve does the temperature remain constant?
A to B
B to C
C to D
E to F
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of energy increases during the flat lines of a heating curve?
Potential energy
Thermal energy
Kinetic energy
Mechanical energy
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does a higher specific heat of a substance indicate about its heating curve?
Irregular slopes
No change in slopes
Shallower slopes
Steeper slopes
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which formula is used to calculate energy change without a phase change?
Q = m * ΔH_vap
Q = m * c * ΔT
Q = m * ΔH
Q = m * ΔH_fus
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the heat of vaporization?
The heat required to melt one gram of a solid
The heat required to freeze one gram of a liquid
The heat required to condense one gram of gas
The heat required to vaporize one gram of liquid
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How much heat is needed to change 250 grams of liquid water into steam at 100 degrees Celsius?
565 joules
5,650 joules
565,000 joules
56,500 joules
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Heat Engines and Refrigeration Concepts
Interactive video
•
9th - 12th Grade

11 questions
Heating and Cooling Curves: Analyzing Temperature Changes During Phase Transitions
Interactive video
•
9th - 12th Grade

11 questions
Understanding the Triple Point and Cyclohexane Experiment
Interactive video
•
9th - 12th Grade

11 questions
Enthalpy Change and Calorimetry Concepts
Interactive video
•
10th - 12th Grade

11 questions
Energy Calculations in Phase Transitions
Interactive video
•
10th - 12th Grade

6 questions
TED-ED: The death of the universe - Ren_e Hlozek
Interactive video
•
KG - University

11 questions
Intermolecular Forces and Phase Changes
Interactive video
•
9th - 12th Grade

11 questions
Heating Curve and Phase Transitions
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Appointment Passes Review
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
Grammar Review
Quiz
•
6th - 9th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade