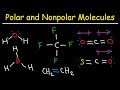
Polar and Non-Polar Molecules
Interactive Video
•
Chemistry, Science
•
9th - 12th Grade
•
Practice Problem
•
Easy
Emma Peterson
Used 9+ times
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first category of non-polar molecules mentioned in the video?
Molecules with hydrogen bonding
Molecules made up of one element
Molecules with asymmetry
Molecules with high electronegativity difference
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is a non-polar molecule made up of only carbon and hydrogen?
Hydrogen fluoride (HF)
Ammonia (NH3)
Methane (CH4)
Water (H2O)
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What characteristic makes carbon tetrafluoride (CF4) a non-polar molecule?
Symmetry of the molecule
Asymmetry of the molecule
High electronegativity difference
Presence of hydrogen bonding
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If the electronegativity difference (EN difference) is less than 0.5, the molecule is generally:
Ionic
Non-polar
Polar
Metallic
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following molecules is non-polar due to a small EN difference?
Iodine monobromide (IBr)
Methanol (CH3OH)
Water (H2O)
Ammonia (NH3)
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of bonding makes a molecule polar?
Metallic bonding
Hydrogen bonding
Van der Waals forces
Ionic bonding
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following molecules is polar due to hydrogen bonding?
Methane (CH4)
Carbon dioxide (CO2)
Oxygen (O2)
Ammonia (NH3)
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

0 questions
Polarity Quiz
Quiz
•

0 questions
Polarity
Quiz
•

0 questions
Intermolecular forces
Quiz
•

0 questions
VSEPR and Polarity (Covalent bonds)
Quiz
•

0 questions
Chemistry: Intermolecular Forces
Quiz
•

0 questions
GREATEST AND LOWEST POLARITY
Quiz
•

0 questions
wavelengths
Quiz
•

0 questions
Polar Bonds
Quiz
•
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

15 questions
4:3 Model Multiplication of Decimals by Whole Numbers
Quiz
•
5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
The Best Christmas Pageant Ever Chapters 1 & 2
Quiz
•
4th Grade

12 questions
Unit 4 Review Day
Quiz
•
3rd Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade
Discover more resources for Chemistry

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Unit 6-Review The Mole
Quiz
•
11th - 12th Grade

29 questions
Physical or Chemical Changes
Quiz
•
9th - 10th Grade

20 questions
Periodic Table & Trends
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

21 questions
Unit 6 -The Mole Review
Quiz
•
11th - 12th Grade

20 questions
Naming Compounds: Basic Ionic and Covalent Naming
Quiz
•
9th - 12th Grade