Exploring Basic Thermochemistry Concepts
Interactive Video
•
Chemistry
•
6th - 10th Grade
•
Practice Problem
•
Hard
Sophia Harris
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the measure of average kinetic energy?
Volume
Pressure
Temperature
Mass
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
At what temperature does all molecular motion stop?
0 Kelvin
100°C
0°C
-273°C
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Can Kelvin temperature be negative?
Yes
No
Only at high pressures
Only in a vacuum
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the opposite of sublimation?
Condensation
Deposition
Evaporation
Melting
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
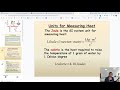
What is the SI unit for measuring heat?
Celsius
Kelvin
Calorie
Joule
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the heat required to raise the temperature of 1g of water by 1°C?
1 Joule
1 Calorie
4.18 Joules
4.18 Calories
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which process involves heat being absorbed?
Exothermic
Endothermic
Isobaric
Isothermal
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
Counting Atoms
Quiz
•
8th Grade

20 questions
Energy Transformations
Quiz
•
9th - 12th Grade

24 questions
Chemical changes
Quiz
•
8th Grade

20 questions
Periodic Table & Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

24 questions
Identifying Types of Chemical Reactions
Quiz
•
10th - 12th Grade

13 questions
Bill Nye - Energy
Interactive video
•
6th Grade