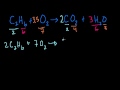
Balancing Chemical Equations Challenge
Interactive Video
•
Chemistry
•
6th - 10th Grade
•
Hard
Aiden Montgomery
Used 1+ times
FREE Resource
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of balancing chemical equations?
To ensure the same number of atoms on both sides of the equation
To make the equation look more complex
To change the properties of the reactants
To identify the type of chemical reaction
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What cannot be altered when balancing a chemical equation?
The physical state of the products
The type of molecules involved
The number of molecules
The relative ratios within a molecule
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in balancing a chemical equation?
Identifying reactants and products
Determining the type of chemical reaction
Balancing single atom molecules
Balancing complex molecules
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the methane combustion example, what is the final step to balance the equation?
Multiply the entire equation to remove fractions
Balance the oxygen atoms
Balance the hydrogen atoms
Adjust the number of carbon atoms
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many molecules of water are produced in the balanced ethane combustion equation?
Seven
Six
Four
Three
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the correct coefficient for diatomic oxygen to balance the ethane combustion equation before removing fractions?
3.5
3
7
14
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the iron oxide and sulfuric acid reaction, what represents the sulfate group for simplification?
X
SO4
O
H2SO4
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Balancing Chemical Equations: Key Concepts and Techniques
Interactive video
•
6th - 10th Grade

11 questions
Balancing Chemical Equations Concepts
Interactive video
•
8th - 10th Grade

11 questions
Photosynthesis and Plant Functions
Interactive video
•
6th - 8th Grade

11 questions
Balancing Chemical Equations
Interactive video
•
8th - 10th Grade

11 questions
Balancing Chemical Equations Practice
Interactive video
•
8th - 10th Grade

7 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

8 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

11 questions
Balancing Chemical Equations: The Inspection Method
Interactive video
•
6th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Physical and Chemical Properties
Quiz
•
8th Grade

20 questions
States of Matter
Quiz
•
8th Grade

20 questions
Counting Atoms Practice
Quiz
•
8th Grade

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

13 questions
Periodic Table of Elements
Lesson
•
8th Grade

20 questions
Chemical Reactions
Quiz
•
8th Grade

15 questions
Valence Electron Practice
Quiz
•
8th Grade

20 questions
Solutes, Solvents, Solutions
Quiz
•
6th - 8th Grade