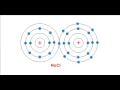
Ionization and Sodium Chloride
Interactive Video
•
Chemistry, Science
•
8th - 10th Grade
•
Medium
Liam Anderson
Used 3+ times
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the process called when molecules split into ions in water?
Ionization
Dissolution
Condensation
Evaporation
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many electrons does a sodium atom have in its outermost orbit?
Eight
Two
One
Seven
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the atomic number of chlorine?
11
17
18
20
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to a sodium atom when it loses its outermost electron?
It becomes a neutron
It remains neutral
It becomes negatively charged
It becomes positively charged
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the common name for sodium chloride?
Vinegar
Table salt
Baking soda
Sugar
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What charge does a chlorine atom acquire when it gains an electron?
No charge
Neutral
Negative
Positive
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why do sodium and chlorine atoms form a stable compound?
They achieve a full outer electron shell
They both gain electrons
They share electrons equally
They lose all their electrons
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Exploring Ionization Energy Trends and Exceptions
Interactive video
•
6th - 10th Grade

11 questions
Ionic and Elementary Charge Concepts
Interactive video
•
9th - 10th Grade

11 questions
Ionization Energy and Periodic Trends Quiz
Interactive video
•
9th - 10th Grade

11 questions
Bonding Basics: Covalent and Ionic Interactions in Chemistry
Interactive video
•
9th - 10th Grade

11 questions
Elements and Compounds: Classifying Matter and Chemical Bonds
Interactive video
•
9th - 10th Grade

11 questions
Periodic Trends and Element Properties
Interactive video
•
9th - 10th Grade

11 questions
Understanding Ionic Compounds
Interactive video
•
7th - 10th Grade

11 questions
Chemical Bonding Concepts
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

20 questions
Physical and Chemical Properties
Quiz
•
8th Grade

20 questions
States of Matter
Quiz
•
8th Grade

20 questions
Counting Atoms Practice
Quiz
•
8th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

20 questions
Solutes, Solvents, Solutions
Quiz
•
6th - 8th Grade